Chemical
Composition
How to Analyse Chemical Composition?
All the carbon (organic) compounds that are present in the
living things are called 'Biomolecules'. As organic molecules, biomolecules
consist primarily of carbon, hydrogen, nitrogen, oxygen and to a smaller
extent, phosphorus and sulfur. Other elements sometimes are incorporated but
are much less common.
However, living organisms also have inorganic elements and
compounds in them. How do we know this? A slightly different but destructive
experiment has to be done. Weigh a small amount of a living tissue (leaf, liver
etc.,) and dry it, so all the water evaporates. The remaining material gives
dry weight. Now if the tissue is fully burnt, all the carbon compounds are
oxidised to gaseous form (CO2, water vapour) and are removed. What
is remaining is called 'ash'. This ash contains inorganic elements (calcium,
magnesium etc.). Inorganic compounds like sulphate, phosphate, etc., are also
seen in the acid-soluble fraction.
Therefore, elemental
analysis gives the elemental composition
of living tissues in the form of hydrogen, oxygen, chlorine, carbon etc., while
analysis for compounds gives an idea of the kind of organic and inorganic
constituents present in living tissues. But, from a biological point of view,
they are classified into amino acids, nucleotide bases, fatty acids etc.
Inorganic Constituents of Living Tissues
|
Component |
Formula |
|
Sodium |
Na+ |
|
Potassium |
K+ |
|
Calcium |
Ca++ |
|
Magnesium |
Mg++ |
|
Water |
H2O |
|
Compounds |
NaCl, CaCO3, P |
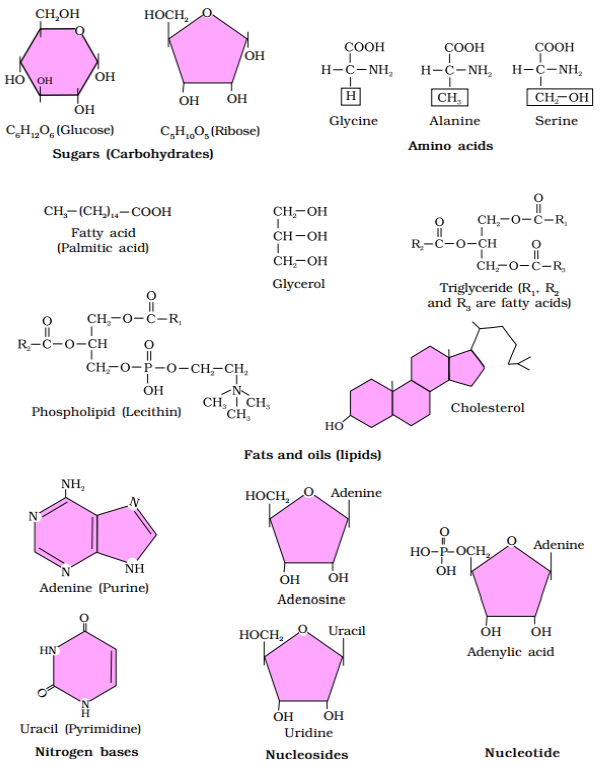
Diagrammatic representation of small
molecular weight organic compounds in living tissues