Enzymes
Enzymes:
Almost
all enzymes are proteins. There are some nucleic acids that exhibit the
characteristics of enzymes, they are called ribozymes. One can depict an enzyme
by a line diagram. An enzyme like any protein has a primary structure, i.e., amino
acid sequence of the protein. An enzyme like any protein has the secondary and
the tertiary structure.

A tertiary structure of proteins
In
tertiary structure (above figure), the backbone of the protein chain folds upon
itself, the chain criss-crosses itself and hence, many crevices or pockets are
made. One such pocket is the ‘active site’. An active site of an enzyme is a
crevice or pocket into which the substrate fits. Thus enzymes, through their
active site, catalyse reactions at a high rate.
Enzyme
catalysts differ from inorganic catalysts in many ways, but one major
difference needs mention. Inorganic catalysts work efficiently at high
temperatures and high pressures, while enzymes get damaged at high temperatures
(say above 40°C). However, enzymes isolated from organisms who normally live
under extremely high temperatures (e.g., hot vents and sulphur springs), are
stable and retain their catalytic power even at high temperatures (upto
80°-90°C). Thermal stability is thus an important quality of such enzymes
isolated from thermophilic organisms.
Chemical Reactions:
Chemical
compounds undergo two types of changes. A physical change simply refers to a
change in shape without breaking of bonds, hence known as a physical process. Another physical process is a
change in state of matter: when ice melts into water, or when water becomes a
vapour. These are also physical processes. However, when bonds are broken and
new bonds are formed during transformation, this will be called a chemical
reaction.
For example:
|
Ba(OH)2
+ H2SO4 → BaSO4 +2H2O |
The above
specified equation is an inorganic chemical reaction. Similarly, hydrolysis of
starch into glucose is an organic chemical reaction. The rate of a physical or chemical process refers to the amount of
product formed per unit time. It can be expressed as:
rate = ![]()
The rate can also be called velocity if the direction
is specified. Rates of physical and chemical processes are majorly influenced by
temperature among other factors. A general rule of thumb is that rate doubles
or decreases by half for every 10°C change in either direction. Catalysed
reactions proceed at rates vastly higher than that of uncatalysed ones. When
enzyme catalysed reactions are observed, the rate would be vastly higher than
the same but uncatalysed reaction.
For
example:
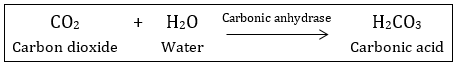
In the
absence of any enzyme, this reaction is
very slow, with about 200 molecules of H2CO3 being formed
in an hour. However, by using the enzyme present within the cytoplasm called
carbonic anhydrase, the reaction speeds dramatically with about 600,000
molecules being formed every second. The enzyme has accelerated the reaction
rate by about 10 million times. The power of enzymes is incredible indeed! There
are thousands of types of enzymes each catalysing a unique chemical or
metabolic reaction. A multistep chemical reaction, when each of the steps is
catalysed by the same enzyme complex or different enzymes, is called a
metabolic pathway.
For example:
|
Glucose |
→ |
2 Pyruvic acid |
||||
|
C6H12O6 |
+ |
O2 |
→ |
2C3H4O3 |
+ |
2H2O |
The above
given equation is actually a metabolic pathway in which glucose becomes pyruvic
acid through ten different enzymes
catalysed metabolic reactions. At this stage,
you should know that this very metabolic pathway with one or two additional
reactions gives rise to a variety of metabolic end products. In our skeletal
muscle, under anaerobic conditions, lactic acid is formed. Under normal aerobic
conditions, pyruvic acid is formed. In yeast, during fermentation, the same
pathway leads to the production of ethanol (alcohol). Hence, in different
conditions different products are possible.
How do
Enzymes bring about such High Rates of Chemical Conversions?
The
chemical or metabolic conversion refers to a reaction. The chemical which is
converted into a product is called a ‘substrate’. Hence enzymes, i.e. proteins
with three dimensional structures including an ‘active site’, convert a
substrate (S) into a product (P).
Symbolically,
this can be depicted as:
|
S → P |
It is now
understood that the substrate ‘S’ has to bind the enzyme at its ‘active site’
within a given cleft or pocket. The substrate has to diffuse towards the ‘active
site’. There is thus, an obligatory formation of an ‘ES’ complex where E stands
for the enzyme. This complex formation is
a transient phenomenon. During the state where the substrate is bound to the enzyme active site, a new structure of
the substrate called transition state structure is formed. Very soon, after the
expected bond breaking/making is completed, the product is released from the
active site.
In other
words, the structure of substrate gets transformed into the structure of the product(s). The pathway of this transformation
must go through the so-called transition state structure. There could be much more ‘altered structural states’ between
the stable substrate and the product. Implicit in this statement is the fact
that all other intermediate structural states are unstable. Stability is
something related to energy status of the molecule or the structure. Hence,
when we look at this pictorially through a graph it looks like something as in
below figure.
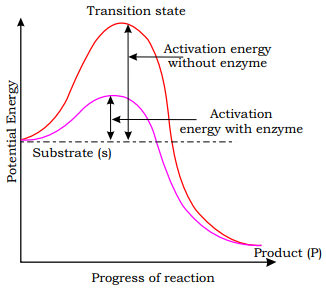
Concept of activation energy
The ![]() -axis
represents the potential energy content. The
-axis
represents the potential energy content. The ![]() -axis
represents the progression of the structural transformation or states through
the ‘transition state’. You would notice two things. The energy level
difference between S and P. If ‘P’ is at a lower level than ‘S’, the reaction
is an exothermic reaction. One need not supply energy (by heating) in order to
form the product. However, whether it is an exothermic or spontaneous reaction
or an endothermic or energy requiring reaction, the ‘S’ has to go through a
much higher energy state or transition state. The difference in average energy
content of ‘S’ from that of this transition state is called ‘activation
energy’.
-axis
represents the progression of the structural transformation or states through
the ‘transition state’. You would notice two things. The energy level
difference between S and P. If ‘P’ is at a lower level than ‘S’, the reaction
is an exothermic reaction. One need not supply energy (by heating) in order to
form the product. However, whether it is an exothermic or spontaneous reaction
or an endothermic or energy requiring reaction, the ‘S’ has to go through a
much higher energy state or transition state. The difference in average energy
content of ‘S’ from that of this transition state is called ‘activation
energy’.
Enzymes
eventually bring down this energy barrier making the transition of ‘S’ to ‘P’ easier.
Nature of Enzyme Action:
Each
enzyme (E) has a substrate (S) binding site in its molecule so that a highly
reactive enzyme-substrate complex (ES) is produced. This complex is short-lived
and dissociates into its product(s) P and the unchanged enzyme with an
intermediate formation of the enzyme-product complex (EP).
The
formation of the ES complex is essential for catalysis.
|
E + S ⇋
ES → EP → E + P |
The
catalytic cycle of an enzyme action can be described in the following steps:
1.
First, the substrate binds to the active site of
the enzyme, fitting into the active site.
2.
The binding of the substrate induces the enzyme to
alter its shape, fitting more tightly around the substrate.
3.
The active site of the enzyme, now in close proximity
of the substrate breaks the chemical bonds of the substrate and the new enzyme-
product complex is formed.
4.
The enzyme releases the products of the reaction
and the free enzyme is ready to bind to another molecule of the substrate and
run through the catalytic cycle once again.
Classification
and Nomenclature of Enzymes:
Thousands
of enzymes have been discovered, isolated and studied. Most of these enzymes
have been classified into different groups based on the type of reactions they
catalyse.
Enzymes
are divided into 6 classes each with 4-13 subclasses and named accordingly by a
four-digit number.
Oxidoreductases/dehydrogenases: Enzymes which catalyse oxidoreduction between two
substrates S and S’.
Example:
|
S reduced + S’ oxidised →
S oxidised + S’ reduced |
Transferases: Enzymes
catalysing a transfer of a group, G (other than hydrogen) between a pair of
substrate S and S’.
Example:
|
S − G + S’ →
S + S’ − G |
Hydrolases: Enzymes catalysing the hydrolysis of ester, ether, peptide, glycosidic,
C-C, C-halide or P-N bonds.
Lyases: Enzymes that catalyse removal of
groups from substrates by mechanisms other than hydrolysis leaving double
bonds.
|
X Y ⎸
⎸ C−C →
X – Y + C = C |
Isomerases: Includes all enzymes catalysing
inter-conversion of optical, geometric or positional isomers.
Ligases: Enzymes catalysing the linking
together of 2 compounds, e.g., enzymes which catalyse joining of C-O, C-S, C-N,
P-O, etc., bonds.