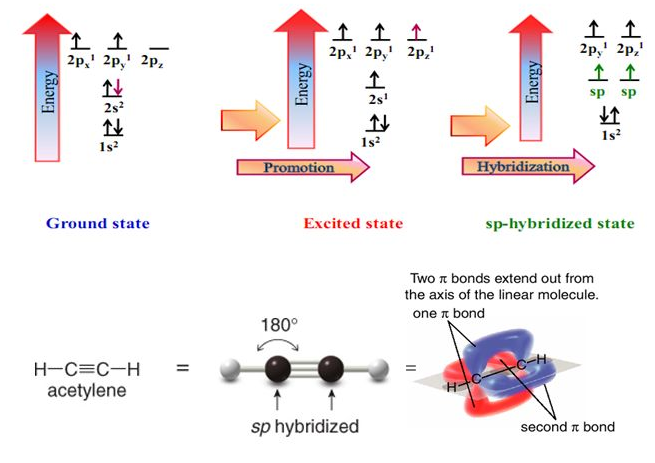
Structure of Triple Bond
Hybridization due to triple bonds allows the uniqueness
of alkyne structure. This triple bond contributes to the nonpolar
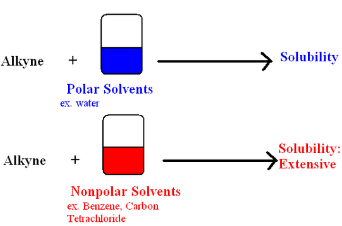
bonding strength, linear, and the acidity of alkynes. Physical Properties include
nonpolar due to slight solubility in polar solvents and insoluble in water.
This solubility in water and polar solvents is a characteristic feature
to alkenes as well. Alkynes dissolve in organic
solvents.
Hybridization
of Alkynes: