Electronic
Configuration of Atoms
Electronic Configuration of
Atoms:
The distribution of electrons into
orbitals of an atom is called its electronic configuration. If one keeps in mind
the basic rules which govern the filling of different atomic orbitals, the
electronic configurations of different atoms can be written very easily.
Example:
Write the electronic configuration for nitrogen which has 7
electrons.
We know that
1s
1
orbital
(2
electrons)
2s 2p
1
orbital 3 orbitals
(2
electrons) (2 electrons each)
|
1s1 |
1s2 |
|
|
|
|
|
|
|
2s1 |
2s2 |
2p1 |
2p2 |
2p3 |
2p4 |
2p5 |
2p6 |
Each orbital contains 2 electrons each. So 1s and 2s get filled completely and 2p
gets partially filled with 3 electrons and 3 places are empty.
We say,
1s and 2s are
filled and 2p is half-filled.
Usually orbitals with
lower principal quantum numbers have lower energies, but that isn't the case
when you compare the energies of the 3d orbitals and the energies of the 4s orbitals. In this case, the 4s orbitals are lower in energy than the
3d orbitals even though they have higher principal quantum numbers.
To check this let us look at Potassium atom which has 19
electrons.
|
1s1 |
1s2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2s1 |
2s2 |
2p1 |
2p2 |
2p3 |
2p4 |
2p5 |
2p6 |
|
|
|
|
|
|
|
|
|
|
|
3s1 |
3s2 |
3p1 |
3p2 |
3p3 |
3p4 |
3p5 |
3p6 |
3d1 |
3d2 |
3d3 |
3d4 |
3d5 |
3d6 |
3d7 |
3d8 |
3d9 |
3d10 |
|
4s1 |
4s2 |
4p1 |
4p2 |
4p3 |
4p4 |
4p5 |
4p6 |
4d1 |
4d2 |
4d3 |
4d4 |
4d5 |
4d6 |
4d7 |
4d8 |
4d9 |
4d10 |
Here,
1s, 2s, 2p, 3s
and 3p get filled completely. There
is one electron left which gets placed in 4s as 4s has a lower energy level
than 3d.
This can be represented by the figure below.
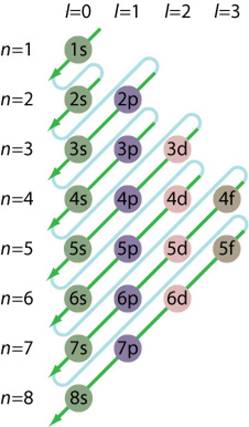
Problems:
1. Write the electronic
configurations of the following ions:
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
Solution:
(a) ![]() ion
ion
The electronic configuration
of H atom is 1s1.
A negative charge on the
species indicates the gain of an electron by it.
∴ Electronic
configuration of ![]() ion = 1s2
ion = 1s2
(b) ![]() ion
ion
The
electronic configuration of F atom is 1s2 2s2 2p5.
A negative
charge on the species indicates the gain of an electron by it.
∴
Electron configuration of ![]() ion = 1s2 2s2 2p6
ion = 1s2 2s2 2p6
(c) ![]() ion
ion
The electronic configuration
of O atom is 1s2 2s2 2p4.
A dinegative
charge on the species indicates that two electrons are gained by it.
∴ Electronic
configuration of ![]() ion = 1s2 2s2 2p6
ion = 1s2 2s2 2p6
2. What are the atomic numbers
of elements whose outermost electrons are represented by
(a) 3s1 (b)
2p3 (c) 3p5
Solution:
(a) 3s1
Completing the electron configuration
of the element as 1s2 2s2 2p6 3s1
∴ Number of electrons
present in the atom of the element
= 2 + 2 + 6 + 1
= 11
∴ Atomic number of the
element = 11
(b) 2p3
Completing the electron
configuration of the element as 1s2 2s2 2p3
∴ Number of electrons
present in the atom of the element
= 2 + 2 + 3
= 7
∴ Atomic number of the
element = 7
(c) 3p5
Completing the electron
configuration of the element as 1s2 2s2 2p5
∴ Number of electrons
present in the atom of the element
= 2 + 2 + 5
= 9
∴ Atomic number of the
element = 9
3. Which atoms are indicated
by the following configurations?
(a) [He] 2s1 (b)
[Ne] 3s2 3p3 (c) [Ar]
4s2 3d1
Solution:
(a) [He] 2s1
The electronic configuration
of the element is [He] 2s1
= 1s2 2s1.
∴ Atomic number of the
element = 3
Hence, the element with the
electronic configuration [He] 2s1 is
lithium (Li).
(b) [Ne] 3s2 3p3
The electronic configuration
of the element is [Ne] 3s2 3p3 = 1s2 2s2 2p6 3s2 3p3.
∴ Atomic number of the
element = 15
Hence, the element with the
electronic configuration [Ne] 3s2 3p3 is phosphorus (P).
(c) [Ar]
4s2 3d1
The electronic configuration of the element is
[Ar] 4s2 3d1 = 1s2 2s2 2p6 3s2 3p6 4s2 3d1.
∴ Atomic number of the
element = 21
Hence, the element with the
electronic configuration [Ar] 4s2 3d1 is
scandium (Sc).