Thermodynamics
It is a well known fact
that most of the physical changes and chemical changes are accompanied by
changes. These energy changes may take place in the form of heat, light, work,
electrical energy, etc & forms of energy are convertible into one another
and hence are related to each other quantitatively.
The branch of science which deals with the
study of different forms of energy and the quantitative relationships between
them is known as thermodynamics.
When we confine our study to chemical
changes and chemical substances only, the restricted branch of thermodynamics
is known as Chemical Thermodynamics.
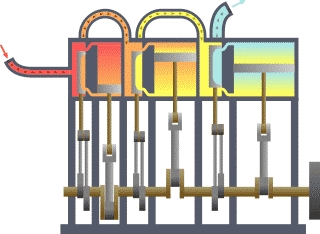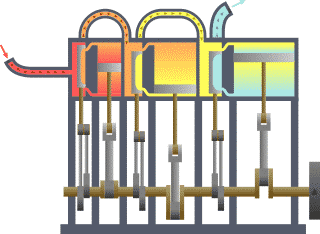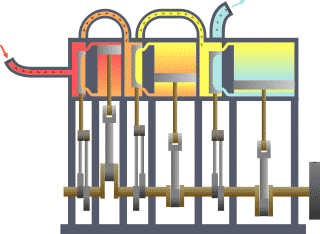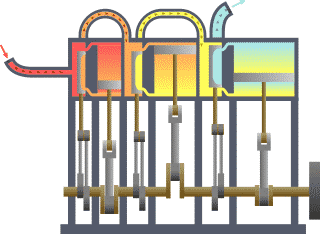
Typical thermodynamic
system - heat moves from hot (boiler) to cold (condenser)
and work is extracted
The complete study of thermodynamics is based upon three generalizations
called First, Second and Third laws of thermodynamics. These laws of
thermodynamics apply only where the system is in equilibrium or moves from one
equilibrium state to another equilibrium state. The laws have been arrived at
purely on the basis of human experience and there is no theoretical proof for
any of these laws. However, the validity of these laws is supported by the fact
that nothing contrary to these laws has been found so far and nothing contrary is
expected.
The importance of
thermodynamics
The importance of
thermodynamics lies in the following facts :-
(i)
It
helps us to predict whether any given chemical reaction can occur under the
given set of conditions.
(ii)
It
helps in predicting the extent of reaction before the equilibrium is attained.
(iii)
It
helps to deduce some important laws like Law of chemical equilibrium,
Distribution law etc.
Problems
1. Which one of the following sets of units represents the
smallest and the largest amount of energy respectively?
a.
eV
and lit atm
b.
erg and cal
c.
cal and eV
d.
lit atm and J
Solution:
1eV=1.6![]() 10-19
J; 1 cal = 4.186
J
10-19
J; 1 cal = 4.186
J
1 erg =10-7 J; 1 lit atm = 101.3 J
Therefore , eV and lit atm represents the smallest and the largest
amount of energy respectively