Acids, Bases and
Salts
Acids,
bases and salts find widespread occurrence in nature. Hydrochloric acid present
in the gastric juice is secreted by the lining of our stomach in a significant
amount of 1.2-1.5 L/day and is essential for digestive processes. Acetic acid
is known to be the main constituent of vinegar. Lemon and orange juices contain
citric and ascorbic acids, and tartaric acid is found in tamarind paste. As
most of the acids taste sour, the word “acid” has been derived from a Latin
word “acidus” meaning sour.
Acids are known to turn blue litmus paper into
red and liberate dihydrogen on reacting with some metals. Similarly, bases are
known to turn red litmus paper blue, taste bitter and feel soapy. When acids
and bases are mixed in the right proportion they react with each other to give
salts.
Ø Arrhenius concept
of acid and bases:
According
to Arrhenius theory, acids are substances that dissociates in water to give
hydrogen ions ![]() and bases are substances that produce hydroxyl
ions
and bases are substances that produce hydroxyl
ions ![]() .
The ionization of an acid
.
The ionization of an acid ![]() can
be represented by the following equations:
can
be represented by the following equations:
![]() →
→ ![]() +
+
![]()
Or
![]() + H2O(l)
→ H3
+ H2O(l)
→ H3![]() +
+ ![]()
A bare proton, H+ is very
reactive and cannot exist freely in aqueous solutions. Thus, it bonds to the
oxygen atom of a solvent water molecule to give trigonal pyramidal hydronium
ion, H3O+ {[H (H2O)]+}
. In this chapter we shall use H+(aq) and H3O+(aq) interchangeably to mean the same i.e., a hydrated
proton. Similarly, a base molecule like
MOH ionizes in aqueous solution according to the equation:
MO![]() →
→ ![]() + O
+ O![]()
The hydroxyl ion also exists in the
hydrated form in the aqueous solution. Arrhenius concept of acid and base,
however, suffers from the limitation of being applicable only to aqueous
solutions and also, does not account for the basicity of substances like,
ammonia which do not possess a hydroxyl group.
Ø Concept of Bronsted – Lowry for acid and base :
Danish
chemist Bronsted and English chemist Lowry presented
the concept of acid and base. They made H (Proton) as a base. According to
their concept, the substance which gives a proton or donates a proton is called
the acid and the substance which receives a proton or accepts a proton is
called the base. Thus, acid is a proton donor and base is a proton acceptor.
Let us take the dissociation reaction of hydrogen chloride in water.
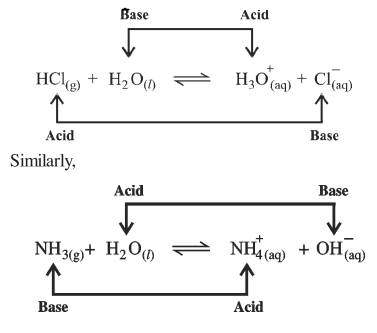
We shall
understand in detail in the first form the above reaction,
|
HCl |
⇋ |
H+ |
+ |
Cl- |
|
Acid – 1 |
|
proton |
|
conjugatebase-1 |
As it gives
proton, HCI is an acid
|
H2O+ |
+ |
H+ |
⇋ |
H3O+ |
|
Base -2 |
|
proton |
|
conjugate acids -2 |
As it accepts a proton, H20
is a base. Total reaction
|
HCl |
+ |
H2O |
⇋ |
H3O(aq) |
+ |
C |
|
Acid -1
|
|
base-2 |
|
acid -2 |
|
base-1 |
Hence, it can
be said that only transfer of proton takes place, it is not obtained free.
Every acid will lose proton and so its conjugate base will be formed and every
base will accept a proton and so its conjugate acid
will be formed. Hence, this concept is known as proton transfer or conjugate
acid-base concept.
|
NH3 |
+ |
H2O |
⇋ |
N |
+ |
O |
|
Base-1 |
|
acid-2 |
|
base-2 |
|
acid-1 |
In the reaction, base NH3
accepts a proton and forms conjugate acid NH4+ ion and
acid H20 loses proton and forms conjugate base OH-.
Thus the concept of Bronsted
- Lowry is found to be more applicable and acceptable than Arrhenius concept.
Even then its limitations are also known. The difficulties are observed in the
study of reactions in organic chemistry and complex salts. BF has no proton
even then it acts as an acid. Hence, the third concept has come in to
existence, which is known as Lewis acid-base concept. Proton is given
importance in Bronsted-Lowry concept.
Ø Lewis concept of
acids and bases:
G.N. Lewis in 1923 defined an acid as
a species which accepts electron pair and base which donates an electron pair.
As far as bases are concerned, there is not much difference between Brönsted-Lowry and Lewis concepts, as the base provides a
lone pair in both the cases. However, in Lewis concept many acids do not have
proton. A typical example is reaction of electron deficient species BF3
with NH3.
BF3
does not have a proton but still acts as an acid and reacts with NH3
by accepting its lone pair of electrons. The reaction can be represented by,
BF3 + :NH3 →
BF3:NH3
Electron deficient species like AlCl3,
Co3+, Mg2+, etc. can act as Lewis acids while species
like H2O, NH3 , OH-
etc. which can donate a pair of electrons, can act as Lewis bases.
Ø Ionisation of acid and base:
Arrhenius concept of acid and base is
useful in understanding ionisation of acid and base
in most
of the chemical and biochemical reactions, ionisation
is there in aqueous medium. Acids like HCIO4, HCl,
HNO3, HBr, H2SO4,
are called strong acids because they are completely ionised
in aqueous solutions. Similarly bases like NaOH, KOH,
Ba(OH)2 , are strong bases because they are
completely ionised in aqueous medium. The magnitude
of ionisation determines the strength of acid or
base. According to Bronsted - Lowry, the magnitude of
accepting or donating a proton delides the strong or
weak (HA) acid or base.
Let us
take the following example:
HA(aq) + H2O(l) ⇋
H3![]() +
+ ![]()
Thus, in above
dissociation, equilibrium is attained and equilibrium is dynamic, that is the
transfer of proton in forward and reverse reaction takes place continuously. If
above reaction is more in forward reaction, then strength of acid will be more
and if in reverse reaction, the strength of acid will be less and so their
respective conjugate base will be weak and strong respectively.
Example: The
conjugate bases of strong acids HCI, H2SO4, HNO3
etc. namely CI- ,S![]() and N
and N![]() will be weak bases. In the same way the
conjugate acids of strong bases NaOH, KOH etc.,
namely Na+ and K+ etc. will be weak acids. Weak acid or
base may not ionise completely and so equilibrium is
obtained. Indicator like phenolphthalein is colourless
in presence of acid and shows pink colour in presence
of base.
will be weak bases. In the same way the
conjugate acids of strong bases NaOH, KOH etc.,
namely Na+ and K+ etc. will be weak acids. Weak acid or
base may not ionise completely and so equilibrium is
obtained. Indicator like phenolphthalein is colourless
in presence of acid and shows pink colour in presence
of base.
Ø
Ionic product of water:
Some substances like water are
unique in their ability of acting both as an acid and a base. We have seen this
in case of water. In presence of an acid, HA it accepts a proton and acts as
the base while in the presence of a base, B- it acts as an acid by
donating a proton. In pure water, one H2O molecule donates proton
and acts as an acid and another water molecules accepts a proton and acts as a
base at the same time.
|
|
+ |
|
⇋ |
|
+ |
OH- |
|
acid |
|
base |
|
Conjugate acid |
|
Conjugate base |
The
dissociation constant is represented by,
K = [H3O+] [OH-]
/ [H2O]
The
concentration of water is omitted from the denominator as water is a pure liquid
and its concentration remains constant. [H2] is incorporated within
the equilibrium constant to give a new constant, Kw, which is called
the ionic product of water.
The
concentration of H+ has been found out experimentally as 1.0 × 10-7
M at 298 K. And, as dissociation of water produces equal number of H+
and OH- ions, the concentration of hydroxyl ions, the concentration
of hydroxyl ions,
[OH-] = [H+] = 1.0 × 10-7
M.
Thus, the
value of Kw at 298K
Kw = [H3O+] [OH-]
= (1.0 × 10-7)2 = 1 × 10-14 M2.
The value
of Kw is temperature dependent as it is an equilibrium constant.
The
density of pure water is 1000 g / L and its molar mass is 18.0 g /mol. From
this the molarity of pure water can be given as,
[H2O] = (1000 g /L)(1
mol/18.0 g) = 55.55 M.
Therefore,
the ratio of dissociated water to that of undissociated
water can be given as:
10-7
/ (55.55) = 1.8 × 10-9 or ˷ 2 in 10-9
We can distinguish acidic, neutral and basic
aqueous solutions by the relative values of the H3O+ and
OH- concentrations:
Acidic:
[H3O+] > [OH-]
Neutral:
[H3O] = [OH-]
Basic:
[H3O+] < [OH-]
Ø pH scale:
If we express the concentration of hydronium ion [H30+]
in molarity then values like 10-12 to 10-2 are possible.
Hence, scientist Sorensen found a scale which is called pH scale. According to
him pH = -log10 [H3O+]. The values 10-12
to 10-2 shown above can be converted to +12 to +2 if calculated on
the basis of this relation and plotting of graph can be easy.
The definition of pH can be
given like this, "pH of a solution is the negative logarithm to the base
10, of the molar concentration of hydrogen or hydronium ion". According to
thermodynamics, activity is more proper word instead of concentration but in
dilute solutions activity and concentration can be considered to be the same.
Now as seen earlier a solution containing 10-7M [H30+]
and [OH-] is neutral. Hence,
pH = -log10 [H3O+]
= -log1010-7 M = 7
and for acidic solution [H30+] > 10-7
M, pH < 7 Similarly, for basic solution [H30+] < 10-7
M, pH > 7, Hence, pH > 7. Therefore, it can be written as:
pH < acidic solution
pH > basic solution
pH = neutral solution
as seen below,
Kw = [H3O+] [OH-]
Putting
the values, Kw =(10-7)(10-7)=
(10-14) and Kw = -![]() )
)
∴
pKw = 14
∴
pH
+ pOH = pKw = 14
Temperature
affects the value of pH, pOH, pKw. The
above discussion can be shown in the following table,
|
Concentration
M |
Acidic |
Neutral
|
Basic |
|
[H3O+] |
More than 10-7 |
10-7 |
Less than 10-7 |
|
[OH-] |
Less than 10-7 |
10-7 |
More than 10-7 |
|
pH |
Less than 107 |
107 |
More than 107 |
|
pOH |
More than 107 |
107 |
More than 107 |