Applications of
Chemical Equilibrium
Some
of the important applications of equilibrium constants are as follows :
·
Prediction of completion of
reaction
·
Prediction about the forward or
reverse direction of the reaction
·
Calculation of equilibrium
constant
Ø Prediction of
completion of reaction:
The value of equilibrium constant shows proportion of completion
of the reaction. If the value of Kc > 1, then more proportion of
products will be obtained, i.e. the reaction of getting products from the
reactants will be towards completion. If
the value of KC is less than 1, then the proportions of reactants
will be more as compared to products and so there will be decrease in the
tendency for the completion of the reaction. If value of KC is 1,
then the proportions of reactants and products will be in equilibrium state.
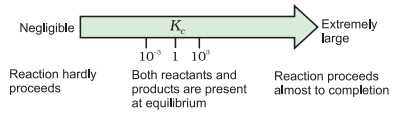
Dependence of extent of reaction
on Kc
Ø Prediction about the
forward or reverse direction of the reaction:
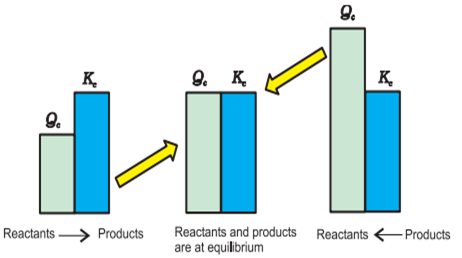
The predictin about
the reaction at any stage will occur in which direction can be decided from the
value of equilibrium constant. For this we have to calculate the reaction
quotient (QC). As the value of Kc for the reaction Aa + Bb ⇋ cC + Dd
Kc
= ![]() = Qc
= Qc
If Qc
< Kc’ or Kc > Qc’ then the reaction will
occur in forward direction, i.e. from reactants to products. If Qc = Kc’ then the reaction will occur
in reverse reaction, i.e. from products to reactants. If Qc = Kc’ , the reaction will remain in equilibrium.
Ø Calculation of
equilibrium constant:
If we know the concentrations of
reactants and products at equilibrium time we can calculate the value of
equilibrium constant and if the initial concentration of the reactants and the value
of equilibrium constant are known, equilibrium concentrations can be
calculated. For this the following steps are to be followed.
Step1: Write chemical reaction in balanced form.
Step 2: (a) Mention the initial concentrations of reactants and
products below them and (b) Mention the changes that take place when
equilibrium is reached. Mention concentration in mollit
-1 or pressure in bar. Write ![]() for the unknown
concentrations and then mention their stoichiometry.
for the unknown
concentrations and then mention their stoichiometry.
Step 3: Put the values of equilibrium concentration in the
equation of chemical equilibrium and solve the equation. If quadratic equation
is obtained, find out the two values using formula for it and determine the
acceptable value.
Step 4: From the value of![]() , determine the acceptable value.
, determine the acceptable value.
Step 5: Verify the value of equilibrium
constant by putting the values of equilibrium concentrations in the equation
for equilibrium constant.
Ø Relationship between
equilibrium constant K, Reaction quotient Q and Gibbs energy G:
The value
of Kc for a reaction does not depend on the rate of the reaction. However, as
you have studied in Unit 6, it is directly related to the thermodynamics of the
reaction and in particular, to the change in Gibbs energy, ∆G. If
·
∆G is negative, then the
reaction is spontaneous and proceeds in the forward direction.
·
∆G is positive, then
reaction is considered non-spontaneous. Instead, as reverse reaction would have
a negative ∆G, the products of the forward reaction shall be converted to
the reactants.
·
∆G is 0, reaction has
achieved equilibrium; at this point, there is no longer any free energy left to
drive the reaction. A mathematical expression of this thermodynamic view of
equilibrium can be described by the following equation:
∆G = ∆G0
+ RT lnQ
Where, G0 is standard Gibbs energy.
At equilibrium, when ∆G
= 0 and Q = Kc’ the equation becomes,
∆G = ∆G0
+ RT ln K = 0
∆G0 = – RT lnK
lnK = – ∆G0
/ RT
Taking antilog of both
sides, we get,
K = ![]()
Hence, using the equation,
the reaction spontaneity can be interpreted in terms of the value of ∆G0.
·
If ∆G0 < 0,
then –∆G0 /RT is positive, and K = ![]() >1, making K >1, which implies a
spontaneous reaction or the reaction which proceeds in the forward direction to
such an extent that the products are present predominantly.
>1, making K >1, which implies a
spontaneous reaction or the reaction which proceeds in the forward direction to
such an extent that the products are present predominantly.
·
If ∆G0 > 0,
then –∆G0 /RT is negative, and K = ![]() <
1, that is , K < 1, which implies a non-spontaneous reaction or a
reaction which proceeds in the forward
direction to such a small degree that only a very minute quantity of product is
formed.
<
1, that is , K < 1, which implies a non-spontaneous reaction or a
reaction which proceeds in the forward
direction to such a small degree that only a very minute quantity of product is
formed.