Factors Affecting Strength of
Acid
If the acid is strong, its value of K will be high and the value
of pH will be low. The dissociation of acid will depend on strength of acid and
the polarity of H-A bond. As the strength of H-A bond decreases, the energy
required for breaking that bond will decrease and HA will be stronger. When
difference between electronegativities of A and B will increase, apparently
ionisation will occur and will be easy to break the structure of the bond.
Hence, acidity will increase.
If the acid is strong, its value of K will be high and the value
of pH will be low. The dissociation of acid will depend on strength of acid and
the polarity of H-A bond. As the strength of H-A bond decreases, the energy
required for breaking that bond will decrease and HA will be stronger. When
difference between electronegativities of A and B will increase, apparently
ionisation will occur and will be easy to break the structure of the bond.
Hence, acidity will increase.

For this
reason, H2S is stronger acid than HO, but if we discuss the elements
in the same period of periodic table, the polarity of H-A bond will determine
the strength of acid. As the electronegativity of A increases, the strength of
acid will increase.
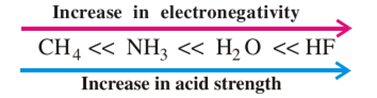
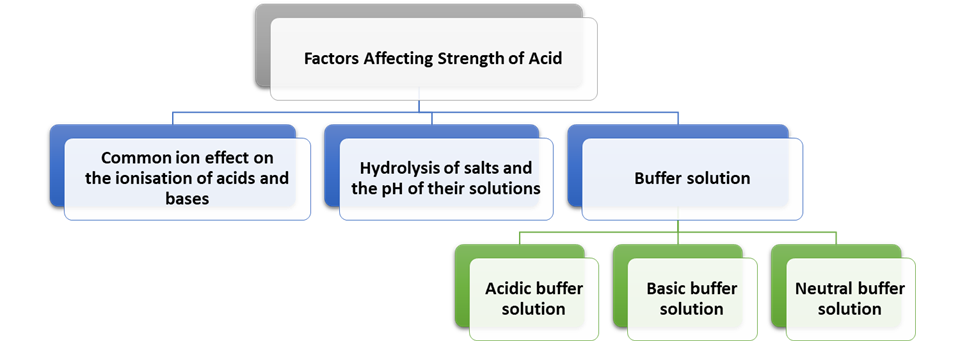
Ř Common ion effect on the ionisation
of acids and bases:
Consider an example of acetic acid dissociation
equilibrium represented as:
C![]() COO
COO![]() ⇋
⇋
![]() + C
+ C![]() CO
CO![]()
or HA![]() ⇋
⇋ ![]() + A
+ A![]()
![]() =
= 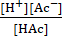
Addition
of acetate ions to an acetic acid solution results in decreasing the
concentration of hydrogen ions, [![]() ].
Also, if H+ ions are added from an external source then the equilibrium moves
in the direction of undissociated acetic acid i.e., in a direction of reducing
the concentration of hydrogen ions, [
].
Also, if H+ ions are added from an external source then the equilibrium moves
in the direction of undissociated acetic acid i.e., in a direction of reducing
the concentration of hydrogen ions, [![]() ]. This
phenomenon is an example of common ion effect. It can be defined as a shift in
equilibrium on adding a substance that provides more of an ionic species
already present in the dissociation equilibrium. Thus, we can say that common
ion effect is a phenomenon based on the Le Chatelier’s principle
]. This
phenomenon is an example of common ion effect. It can be defined as a shift in
equilibrium on adding a substance that provides more of an ionic species
already present in the dissociation equilibrium. Thus, we can say that common
ion effect is a phenomenon based on the Le Chatelier’s principle
In
order to evaluate the pH of the solution resulting on addition of 0.05M acetate
ion to 0.05M acetic acid solution, we shall consider the acetic acid
dissociation equilibrium once again,
HA![]() ⇋
⇋
![]() + A
+ A![]()
Initial concentration (M)
0.05 0 0.05
Let x be the extent of
ionization of acetic acid.
Change in concentration (M)
–x +x +x
Equilibrium concentration (M)
0.05-x x 0.05+x
Therefore, ![]() = [
= [![]() ][A
][A![]() ]/[H
Ac] = {(0.05+x)(x)}/(0.05-x)
]/[H
Ac] = {(0.05+x)(x)}/(0.05-x)
As ![]() is small for a very weak acid,
is small for a very weak acid, ![]() <<0.05.
<<0.05.
Hence, (0.05 + ![]() ) ≈
(0.05 –
) ≈
(0.05 – ![]() ) ≈
0.05
) ≈
0.05
Thus,
1.8 × ![]() =
= ![]()
= ![]()
= ![]() = [
= [![]() ] = 1.8
×
] = 1.8
× ![]() M
M
pH = – log(1.8 × ![]() ) =
4.74
) =
4.74
Ř Hydrolysis of salts and the
pH of their solutions:
Salt is
obtained by combination of acid and base in definite proportion. When the salt
is dissolved in water, ionisation occurs. The following types of salts are
obtained depending on acid or base strong or weak. Salt formed from strong base
and strong acid is neutral and so its pH is 7.0. e.g., Nacl.
But if
salt is formed from strong acid and weak base, it will be acidic and pH of its aqueous
solution will be less than 7 e.g. N![]() Cl.
Similarly, salt formed from weak acid and strong base is basic and its pH in
aqueous solution will be more 7, e.g. C
Cl.
Similarly, salt formed from weak acid and strong base is basic and its pH in
aqueous solution will be more 7, e.g. C![]() COONa.
The reason is that salt reacts with water and undergoes hydrolysis reaction.
COONa.
The reason is that salt reacts with water and undergoes hydrolysis reaction.
|
Acid |
Base |
Salt |
Property |
Example |
|
Strong |
Strong
|
Neutral |
Neutral |
NaOH
+ HCl → NaCl + |
|
Strong |
Weak |
Acidic |
Acidic |
HCl +
N |
|
Weak |
Strong |
Basic |
Basic |
C |
|
Weak |
Weak |
Neutral Or Acidic
Or basic |
Neutral Or Acidic Or basic |
C HCOOH
+ N
|
Hydrolysis reaction is an equilibrium
reaction and so its corresponding equilibrium constant can be calculated which is
known as hydrolysis constant (![]() ). It can be determined with the help
of the example of weak acid and strong base e.g. C
). It can be determined with the help
of the example of weak acid and strong base e.g. C![]() COONa
COONa
(1)
For salt of weak acid and strong base:
C![]() COON
COON![]() +
+ ![]() ⇋ C
⇋ C![]() COO
COO![]() + NaO
+ NaO![]()
![]() =
= ![]()
(2)
For salt of strong acid and weak base:
N![]() C
C![]() +
+ ![]() ⇋ NaO
⇋ NaO![]() + HC
+ HC![]()
![]() =
= ![]()
(3)
For salt of weak acid and weak base:
C![]() COON
COON![]() +
+ ![]() ⇋ C
⇋ C![]() COO
COO![]() + N
+ N![]() O
O![]()
![]() =
= ![]()
Earlier you have studied about K and K. The equation of K. can be
obtained from them as shown below :
|
Salt |
Hydrolysis of water |
pH of solution |
|
Strong
acid weak base |
|
>7 |
|
Weak
acid strong base |
|
<
7 |
|
Weak acid
weak base |
|
=7 |
Thus, from the nature of the salt pH of its aqueous solution can
be calculated.
Ř Buffer solution:
The pH of the fluids like blood in our
body and urine is almost constant. If there is change in this pH, it affects
biochemical reaction in the body. The pH of chemical and biochemical reactions
in our body are constant, viz. the pH of human saliva is 6.4. In addition, hydrochloric acid is present in human
stomach which helps in digestion. The pH of cosmetics are also kept constant.
Hence, the question arises that how pH in any solution can be kept constant.
Such solutions are called buffer solutions. Its definition can be given as
below:
"The solution which resists the
change in pH carried out by addition of acid or base in small proportion to
them or are being diluted, and the values of their pH remain constant are
called buffer solutions". Buffer solutions can be acidic or basic. If p![]() of weak acid and p
of weak acid and p![]() of weak base are known, buffer solutions of
known pH can be prepared. Buffer solutions can be of three types as follows:
of weak base are known, buffer solutions of
known pH can be prepared. Buffer solutions can be of three types as follows:
(i)
Acidic buffer
solution :
Acidic buffer solution can be prepared
by mixture of weak acid and its salt with strong base.
(ii)
Basic buffer solution :
Basic buffer solution can be prepared by mixture of
weak base and its salt with strong acid.
(iii)
Neutral buffer
solution :
Neutral buffer solution can be
prepared by neutralisation of weak acid and weak base. These types of buffer
solutions are shown below:
|
Type |
Substance |
Value of pH |
|
Acidic |
C |
<7 |
|
Basic |
N |
>7 |
|
Neutral
|
C |
≈7 |
Buffer
solution of known pH can be prepared by using the following Henderson-
Hasselbalch equation.
For acidic solution,
pH = p![]() +
+ ![]()
Where
[acid] is concentration of weak acid and its dissociation constant is ![]() and [salt] is concentration of the salt of
this weak acid with strong base. For an acidic buffer solution it can be
written as
and [salt] is concentration of the salt of
this weak acid with strong base. For an acidic buffer solution it can be
written as
pH = p![]() +
+ ![]()
Similarly, for basic buffer solution e.g.
N![]() OH
+
OH
+ ![]() can be written that
can be written that
pH = p![]() +
+ ![]()
Such buffer solutions can be used in
chemical and biochemical reactions and especially in analytical chemistry. In
human body buffer solutions containing [HC![]() ] and [C
] and [C![]() ] as well as
[
] as well as
[![]() P
P![]() ] and [HP
] and [HP![]() ] are present.
] are present.