Redox Reactions and Oxidation Number
Redox Reactions:
Redox Reactions is reaction
in which oxidation and reduction both are going to happen, called redox
reaction. It may be in any medium (acidic, neutral, and basic).
Redox
Reaction in terms of electron transfer reactions:
We have already learnt that the reactions
2Na(s) + Cl2 (g) →
2NaCl (s)
4Na(s) + O2 (g) →
2Na2O(s)
2Na(s) + S(s) → Na2S(s)
These are redox reactions
because in each of these reactions sodium is oxidised due to the addition of
either oxygen or more electronegative element to sodium. Simultaneously,
chlorine, oxygen and sulphur are reduced because to each of these, the
electropositive element sodium has been added. We know that sodium chloride,
sodium oxide and sodium sulphide are ionic compounds and perhaps better written
as Na+Cl(s), (Na+ )2O2(s), and
(Na+ )2 S2(s).
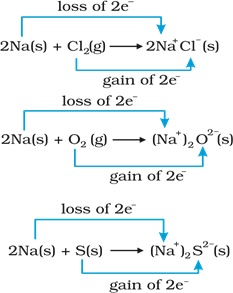
For convenience, each of the above processes can be
considered as two separate steps, one involving the loss of electrons and the
other the gain of electrons.
2 Na(s) → 2 Na+
(g) + 2e
Cl2 (g) + 2e
→ 2 Cl (g)
Each of the above steps is called a half reaction. First is
called half oxidation reaction other is called half reduction reaction. The
final reactions that involve loss of electrons are called oxidation reactions.
Similarly, the half reactions that involve gain of electrons are called
reduction reaction.
Definitions:
Oxidation: Loss of electrons(s) by any
species.
Reduction: Gain of electron(s) by any
species.
Oxidising
agent: It
accepts electron to each of the elements interacting with it and thus help in
oxidising them.
Reducing
agent: It
donates electron to each of the element interacting with it and thus help in reducing them.
Oxidation
Number:
Oxidation number denotes the oxidation state of an element in
a compound. The oxidation number of an atom is the charge that atom would have
if the compound was composed of ions.
We have seen electron transfers in a reaction which is ionic
reaction. But if a reaction has covalent properties then they can transfer
their whole electron and that method we cant apply. So, we have to find out
the oxidation state of the element to understand the partial sharing electron
in covalent reaction.
There are some rules to find out the oxidation state of an
element which are given below: -
1. The
oxidation number of an atom is zero in a neutral substance that contains atoms
of only one element. Thus, the atoms in O2, O3, P4,
S8, and aluminium metal all have an oxidation number of 0.
2. The
oxidation number of simple ions is equal to the charge on the ion. The
oxidation number of sodium in the Na+ ion is +1, for example,
and the oxidation number of chlorine in the Cl- ion is -1.
3. The
oxidation number of hydrogen is +1 when it is combined with a non-metal as in
CH4, NH3, H2O, and HCl.
4. The
oxidation number of hydrogen is -1 when it is combined with a metal as in. LiH,
NaH, CaH2, and LiAlH4.
5. The
metals in Group IA form compounds (such as Li3N and Na2S)
in which the metal atom has an oxidation number of +1
6. The
elements in Group IIA form compounds (such as Mg3N2 and
CaCO3) in which the metal atom has a +2-oxidation number.
7. Oxygen
usually has an oxidation number of -2. Exceptions include molecules and
polyatomic ions that contain O-O bonds, such as O2, O3, H2O2,
and the ![]() ion.
ion.
8. The
elements in Group VIIA often form compounds (such as AlF3, HCl, and ZnBr2) in which the non-metal has a -1
oxidation number.
9. The sum
of the oxidation numbers in a neutral compound is zero.
H2O:
2(+1) + (-2) = 0
10.
The sum of the oxidation numbers in a polyatomic
ion is equal to the charge on the ion. The oxidation number of the sulfur atom in the SO42- ion
must be +6, for example, because the sum of the oxidation numbers of the atoms
in this ion must equal -2.
SO42-:
(+6) + 4(-2) = -2
11.
Elements toward the bottom left corner of the
periodic table are more likely to have positive oxidation numbers than those
toward the upper right corner of the table. Sulphur has a positive oxidation
number in SO2, for example, because it is below oxygen in the periodic
table.
SO2:
(+4) + 2(-2) = 0
Definitions:
Oxidation: An increase in the oxidation
number of the electron in the given substance.
Reduction: A decrease in the oxidation
number of the element in the given substance.
Oxidising
agent: A
reagent which can increase the oxidation number of an element in a given
substance. These reagents are called as oxidants also.
Reducing
agent: A
reagent which lowers the oxidation number of an element in a given substance.
These reagents ae also called as reducants.
Redox
reactions: Reactions
which involve change in oxidation number of the interacting species.
Types
of Redox Reactions:
1.
Combination reaction:
A combination reaction may
be denoted in the manner:
A + B → C
Either A and B or both A and B must be in the elemental form
for such a reaction to be a redox reaction. All combustion reactions, which
make use of elemental dioxygen, as well as other reactions involving elements
other than dioxygen, are redox reactions. Some important examples of this
category are:
C(s) + O2 (g) ![]() CO2 (g)
CO2 (g)
CH4 (g) + 2O2
(g) ![]() CO2 (g) + 2H2O(l)
CO2 (g) + 2H2O(l)
2.
Decomposition reactions
Decomposition reactions are
the opposite of combination reactions. A decomposition reaction leads to the
breakdown of a compound into two or more components at least one of which must
be in the elemental state.
2H2O (l) ![]() 2H2 (g) + O2 (g)
2H2 (g) + O2 (g)
2KClO3 (s) ![]() 2KCl (s) + 3O2 (g)
2KCl (s) + 3O2 (g)
All decomposition reactions are not redox reaction. For
example, decomposition of calcium carbonate is not a redox reaction
CaCO3 (s) ![]() CaO(s) + CO2
(g)
CaO(s) + CO2
(g)
3.
Displacement reaction
Displacement reactions, also known as replacement reactions,
involve compounds and the replacing of elements. They occur as single and
double replacement reactions. In a displacement reaction, an ion in a compound
is replaced by an ion of another element. It may be denoted as:
X + YZ → XZ + Y
Displacement reactions fit into two categories: metal
displacement and non-metal displacement.
(a)
Metal displacement:
A metal in a compound can be
displaced by another metal in the uncombine state.
Metal displacement
reactions find many applications in metallurgical processes in which pure
metals are obtained from their compounds in ores.
CuSO4 (aq)
+ Zn (s) → Cu(s) + ZnSO4 (aq)
V2O5
(s) + 5Ca (s) ![]() 2V (s) + 5CaO (s)
2V (s) + 5CaO (s)
Cr2O3
(s) + 2 Al (s) ![]() Al2O3 (s) + 2Cr(s)
Al2O3 (s) + 2Cr(s)
In each case, the reducing metal is a better reducing agent
than the one that is being reduced which evidently shows more capability to
lose electrons as compared to the one that is reduced.
(b) Non-metal
displacement:
The non-metal displacement
redox reactions include hydrogen displacement and a rarely occurring reaction
involving oxygen displacement.
·
All alkali metals and some alkaline earth metals
(Ca, Sr, and Ba) which are very good reductants, will
displace hydrogen from cold water.
2Na(s) + 2H2O(l)
→ 2NaOH(aq) + H2(g)
Ca(s) + 2H2O(l)
→ Ca(OH)2 (aq) + H2 (g)
·
Less active metals such as magnesium and iron
react with steam to produce dihydrogen gas:
Mg(s) + 2H2O(l) ![]() Mg(OH)2 (s) + H2
(g)
Mg(OH)2 (s) + H2
(g)
2Fe(s) + 3H2O(l) ![]() Fe2O3(s) + 3H2(g)
Fe2O3(s) + 3H2(g)
·
Many metals, including those which do not react
with cold water, are capable of displacing hydrogen from acids. Dihydrogen from
acids may even be produced by such metals which do not react with steam.
Cadmium and tin are the examples of such metals.
Zn(s) + 2HCl(aq) → ZnCl2
(aq) + H2 (g)
Mg (s) +
2HCl (aq) → MgCl2 (aq) + H2 (g)
·
Fluorine is so reactive that it can replace
chloride, bromide and iodide ions in solution. In fact, fluorine is so reactive
that it attacks water and displaces the oxygen of water:
2H2O
(l) + 2F2 (g) → 4HF(aq) + O2 (g)
·
It is for this reason that the displacement
reactions of chlorine, bromine and iodine using fluorine are not generally
carried out in aqueous solution. On the other hand, chlorine can displace
bromide and iodide ions in an aqueous solution as shown below:
Cl2
(g) + 2KBr (aq) → 2 KCl
(aq) + Br2 (l)
Cl2
(g) + 2KI (aq) → 2 KCl
(aq) + I2 (s)
·
As Br2 and I2 are coloured
and dissolve in CCl4 , can easily be
identified from the colour of the solution. The above reactions can be written
in ionic form as:
Cl2
(g) + 2Br (aq) → 2Cl (aq) + Br2 (l)
Cl2
(g) + 2I (aq) → 2Cl (aq) + I2 (s)
·
The halogen displacement reactions have a direct
industrial application. The recovery of halogens from their halides requires an
oxidation process, which is represented by:
2X
→ X2 + 2e
Here, X
denotes a halogen element. Whereas chemical means are available to oxidise Cl ,
Br and I-, as fluorine is the strongest oxidising agent;
there is no way to convert F ions to F2 by chemical
means. The only way to achieve F2 from F is to oxidise electrolytically.
4.
Disproportionation reaction
In some redox reactions,
substances can be both oxidized and reduced. These are known as
disproportionation reactions. One real-life example of such a process is the
reaction of hydrogen peroxide, H2O2, when it is poured
over a wound. At first, this might look like a simple decomposition reaction,
because hydrogen peroxide breaks down to produce oxygen and water:
2H2O2(aq) → 2 H2O(l)
+ O2(g)
The key to this reaction lies in the oxidation states of
oxygen, however. Notice that oxygen is present in the reactant and both products. In H2O2,
oxygen has an oxidation state of -1. In H2O, its oxidation state is
-2, and it has been reduced. In O2 however, its oxidation state
is 0, and it has been oxidized. Oxygen has been both oxidized and reduced in
the reaction, making this a disproportionation reaction. The general form for
this reaction is as follows:
2A → A + A
The
paradox of Fractional Oxidation Number:
We have discussed that oxidation number can be positive,
negative and zero also. But sometimes, we come across with certain compounds in
which the oxidation number of a particular element in the compound is in
fraction not in integer. There are some Examples:
C3O2 [where oxidation number of
carbon is ![]() ],
],
Br3O8 [where oxidation number of
bromine is ![]() ]
]
Na2S4O6 (where
oxidation number of sulphur is 2.5)
As we know that electrons are never shared/transferred in
fraction. It is done by integers only. In actual, this fraction oxidation state
is the average oxidation state of the element under examination and the
structural parameters reveal that the element for whom fractional oxidation
state is realized is present in different oxidation states.
We can explain this rule by their structure which are given
below-
O = C = C*= C = O
(structure of carbon suboxide)
Carbon oxide has three carbon and among them two carbon have
similar properties and other (starred carbon) has different properties. (Here,
properties in a sense, we are talking about oxidation number)
Oxidation state of without star carbon = +2
Oxidation sate of starred carbon = 0
The average oxidation number of carbon = ![]() =
= ![]()
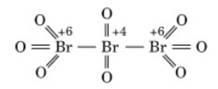
(structure of tribromooctaoxide)
It contains three bromine and in them two are same oxidation
number and other has different oxidation number.
Oxidation state of first and last bromine in structure
= +6
Oxidation state of middle bromine in structure = +4
The average oxidation state of bromine = ![]() =
= ![]()
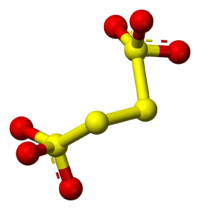
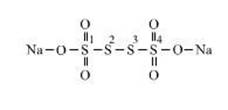
Structure of sodium tetrathionate
has four Sulphur but they are two type of Sulphur in that. First and fourth
Sulphur have same properties, and second and third have different properties
from first and fourth Sulphur.
Oxidation state of first and fourth Sulphur is = +5
Oxidation state of second and third Sulphur is = 0
The average oxidation state of Sulphur is = ![]() = 2.5
= 2.5
We can conclude that the idea of fractional oxidation state
should be taken with care and the reality is revealed by the structure only.
Whenever we come across with fractional oxidation state of any particular
element in any species, we must understand that this is the average oxidation
number only. However, the oxidation state may be in fraction as in O2+
and O2- where it is +1/2 and -1/2 respectively.