Voids
Void or Space or Holes:
Empty
or vacant space present between spheres of a unit cell, is called void or space
or hole or interstitial void. When particles are closed packed resulting in
either cpp or hcp
structure, two types of voids are generated:
·
Tetrahedral voids
·
Octahedral voids
Tetrahedral voids:
Tetrahedral voids are holes or voids surrounded
by four spheres present at the corner of a tetrahedron. Coordination number of
a tetrahedral void is 4.

R(void)=0.255 ×
R(sphere)
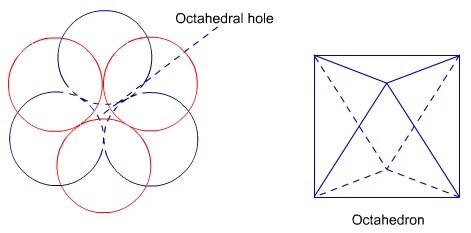
Octahedral voids:
Octahedral voids are holes surrounded by six
spheres located on a regular tetrahedron. Coordination number of octahedral
void is 6.
R(void)=0.414
× R(sphere)