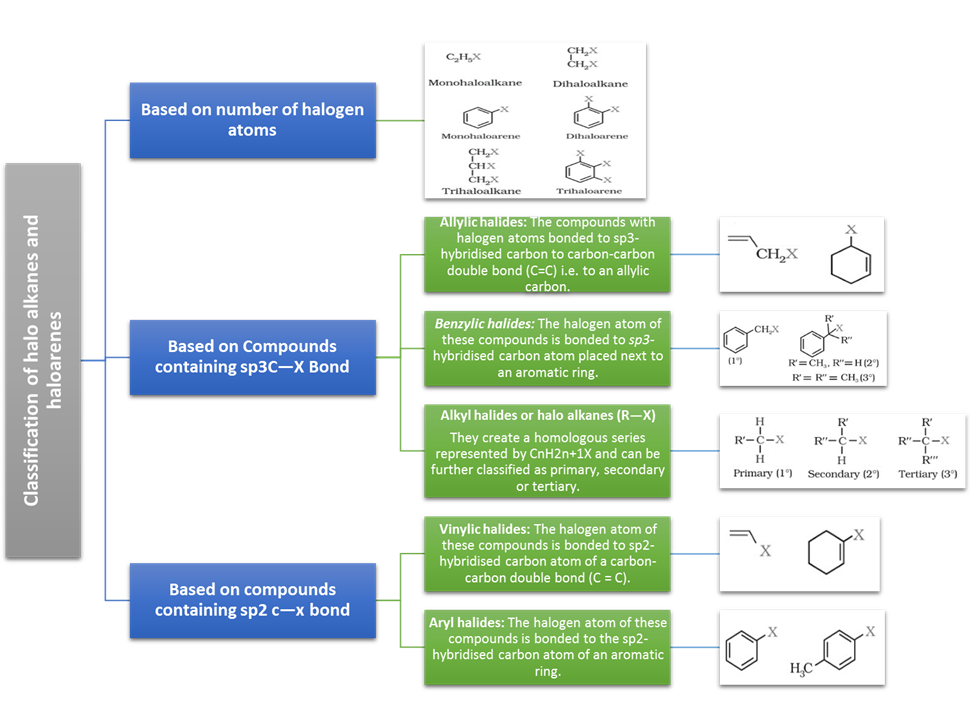
Classification
Haloalkanes and haloarenes may
be classified as follows:
On the Basis of Number of Halogen Atoms:
These may be classified as mono, di, or polyhalogen (tri-,tetra-, etc.) compounds
depending on whether they contain one, two or more halogen atoms in their
structures. For example,
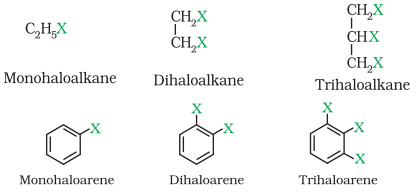
Monohalocompounds may further be classified according
to the hybridisation of the carbon atom to which the halogen is bonded.
Compounds Containing sp3 C—X Bond (X= F, Cl, Br,
I)
This class includes:
(a) Alkyl halides or haloalkanes
(R—X)
In alkyl halides, the halogen atom is bonded to
an alkyl group (R). They form a homologous series represented by CnH2n+1X.
They are further classified as primary, secondary or tertiary according to the
nature of carbon to which halogen is attached.
If halogen is attached to a primary carbon atom
in an alkyl halide, the alkyl halide is called primary alkyl halide or 1° alkyl
halide.
Similarly, if halogen is attached to secondary
or tertiary carbon atom, the alkyl halide is called secondary alkyl halide (2°)
and tertiary (3°) alkyl halide, respectively.
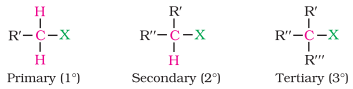
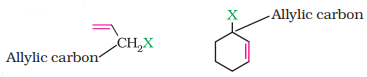
(b) Allylic halides
These are the compounds in which the halogen
atom is bonded to an sp3 -hybridised carbon atom adjacent to carbon-carbon
double bond (C=C) i.e. to an allylic carbon.
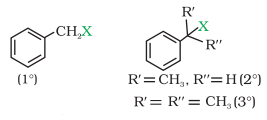
(c) Benzylic halides
These are the compounds in which the halogen
atom is bonded to an sp3 -hybridised carbon atom attached to an aromatic
ring.
Compounds Containing
sp2 C—X Bond
This class includes:
(a) Vinylic
halides
These are the compounds in which the halogen
atom is bonded to a sp2
-hybridised carbon atom of a carbon-carbon double bond (C = C).

(b) Aryl halides
These are the compounds in which the halogen atom
is directly bonded to the sp2
-hybridised carbon atom of an aromatic ring.