The process of vulcanisation makes rubber
- Soluble in water
- Elastic
- Hard
- Soft
If are the number of molecules with molecular masses respectively, then average molecular mass is expressed as
-
-
- and
- None of these
Buna-N synthetic rubber is a copolymer if:
-
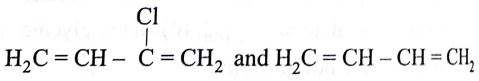
-
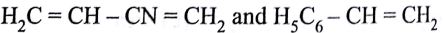
-
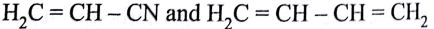
-
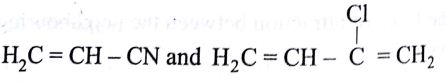
Given the polymers,
A = Nylon-6,6; B = Buna-S; C = Polythene
Arrange these in descending order of their intermolecular forces:
- C < B > A
- B > C > A
- B < C < A
- C < A < B
The species which can best server as an initiator for the cationic polymerization is:
-
-
- BuLi
-
Which one of the following is not a condensation polymer?
- Nylon-6,6
- Nylon-6
- Dacron
- Buna-S
A thermoplastic among the following is:
- Bakelite
- Polystyrene
- Terylene
- Urea formaldehyde resin
In Buna-S symbol 'Bu' stands for:
- I-butene
- 2-butene
- n-butene
- Butadiene
Plexiglass is
- PAN
- Poly(ethyl acrylate)
- Poly(methyl methacrylate)
- None of these
Which is an example of thermosetting polymer?
- Polythene
- PVC
- Teflon
- Bakelite
Which of the following is a semisynthetic polymer?
- Silk
- Wood
- Rayon
- Natural rubber
The molecular formula of hexamethylene diammine adipate(monomer of nylon-66) is
-
-
-
-
Which of the following rubber is not a polydiene?
- Polysisaprene
- Polychloroprene
- Thiokol rubber
- Nitrile rubber
Discovery of 'nylon' is associated with
- New York and London
- New York and Longuet
- Nyholm and London
- None of these
Which of the following is a synthetic polymer?
- Rubber
- Perspex
- Protein
- Cellulose
Which of the following is fully fluorinated polymer?
- Teflon
- Neoprene
- Thiokol
- PVC
The polymer containing strong intermolecular forces, eg hydrogen bonding is:
- Natural rubber
- Teflon
- Nylon-6,6
- Polystyrene
Polymer used in bullet proof glass is:
- PMMA
- Lexan
- Normex
- Kevlar
Natural polymer among the following is:
- Nylon
- Glyptal
- Cellulose
- Terylene
Which of the following is a chain-growth polymer?
- Strach
- Nucleic acid
- Polystyrene
- Protein
Which of the following is a biodegradable polymer?
- Polythene
- PVC
- Bakelite
- PHBV
'Rayon' is
- Natural silk
- Artificial silk
- Natural plastic or rubber
- Synthetic plastic
Neoprene, a synthetic rubber contains which of the following element besides C and H
- N
- O
- Cl
- F
Nylon-6,6 is a
- Natural polymer
- Condensation polymer
- Addition polymer
- Substitution polymer
A condensation polymer among the following polymers is
- PVC
- Teflon
- Decron
- Polystyrene
Which of the following is not a natural polymer?
- Cellulose
- Protein
- PVC
- Nucleic acid
Which of the following is not correct regarding terylene?
- Step-growth polymer
- Synthetic fibre
- Thermosetting plastic
- It is also called decron
Isoprene is a valuable substance for making
- Propene
- Liquid fuel
- Synthetic rubber
- Petrol
Teflon is a polymer of
- Tetrafluoro ethane
- Tetrafluro propene
- Difluorodichloro ethane
- Difluoro ethane
Which of the following is used in vulcanization of rubber?
-
-
-
-
Which of the following statements is not correct?
- Caprolactum is the monomer of nylon-6
- Terylene is a polyester polymer
- Phenol formaldehyde resin is known as bakelite
- The monomer of natural rubber is butadiene
Which of the following is an example of copolymer?
- Teflon
- Buna-S
- PVC
- Polypropylene
Which is not a polymer?
- Sucrose
- Enzyme
- Starch
- Teflon
Which one of the following is used to make 'non-stick' cookware?
- PVC
- Polystyrene
- Polyethylene terephthalate
- Polytetrafluoroethylene
Characteristic property of teflon is
- 2000 poise viscosity
- High surface tension
- Non-inflammable and resistant to heat
- Highly reactive
Which of the following is not a polymer?
- Silk
- DNA
- DDT
- Starch
Polythene is
- Thermoplastic
- Thermosetting
- Both Thermoplastic and Thermosetting
- None of these
Bakelites are
- Rubber
- Rayon
- Resins
- Plasticisers
Which of the following is a step-growth polymer?
- Polyisoprene
- Polythene
- Nylon
- Polyacrylonitrile
In the manufacture of polythene by the Ziegler process using ethylene, the temperature for proper polymerisation required is
- Below
-
-
-
Styrene at room temperature is
- Solid
- Liquid
- Gas
- Colloidal solution
The ziegler-Natta catalysts are
- Stereospecific
- Non metallic complexes
- Gaseous catalysts
- Universal in all polymerisation reactions
Melamine is
- Gas
- Yellow liqiud
- White crystalline solid
- Colloidal solution
Glyptal is a
- Viscose rayon
- Nylon
- Polystyrene
- Alkyd resin
Which of the following is not polyamide?
- Nylon-66
- Protein
- Glyptal
- Nylon-6
Which of the following is a copolymer formed by condensation polymeraization?
- Buna-S rubber
- Buna-N
- Neoprene
- Terylene
Among cellulose,poly(vinyl chloride),nylon and natural rubber, the polymer in which the intermolecular force of attraction is weakest is:
- Nylon
- Poly (Vinyl chloride)
- Cellulose
- Natural rubber
Which of the following statements is false?
- Artificial silk is derived from cellulose
- Nylon-66 is an example of elastomer
- The repeat unit in natural rubber is isoprene
- Both starch and cellulose are polymers of glucose
What is not true about polymers?
- Polymers do not carry any charge
- Polymers have high viscosity
- Polymers scatter light
- Polymers have low molecular weight
Which of the following is synthetic rubber?
- Buna-S
- Neoprene
- Both Buna-S and Neoprene
- None of these
Which of the following is not an example of natural polymer?
- Wool
- Silk
- Leather
- Nylon
Acetate rayon is prepared from
- Acetic acid
- Glycerol
- Starch
- Cellulose
Dacron is an example of
- Polyamide
- Polypropylene
- Polyurethane
- Polyester
Which of the following is a thermosetting polymer?
- Nylon-6
- Nylon-6,6
- Bakelite
- SBR
Assertion: Terylene is a condensation polymer.
Reason: Terylene is formed by ethylene glycol and terephthalic acid with elimination of water molecule.
- Both the assertion and reason are true and reason is a true explanation of the assertion.
- Both the assertion and reason are true but the reason is not the correct explanation of asserstion.
- The assertion is true but reason is false.
- Assertion is false but the reason is true.
Assertion: Cellulose acetate is a semisynthetic polymer.
Reason: Chemical name of cellulose acetate polymer is rayon.
- Both the assertion and reason are true and reason is a true explanation of the assertion.
- Both the assertion and reason are true but the reason is not the correct explanation of asserstion.
- The assertion is true but reason is false.
- Assertion is false but the reason is true.
Assertion: Nylon-6 is a synthetic polymer formed by hydrolysis of caprolactam.
Reason: Nylon-6 is used in tyre cords.
- Both the assertion and reason are true and reason is a true explanation of the assertion.
- Both the assertion and reason are true but the reason is not the correct explanation of asserstion.
- The assertion is true but reason is false.
- Assertion is false but the reason is true.
Assertion: Phenol formaldehyde polymer is a thermosetting polymer.
Reason: Bakelite can be remoulded again in desired shape by fusion.
- Both the assertion and reason are true and reason is a true explanation of the assertion.
- Both the assertion and reason are true but the reason is not the correct explanation of asserstion.
- The assertion is true but reason is false.
- Assertion is false but the reason is true.
Which one is chain growth polymer?
(AIPMT/NEET-2004)
- Starch
- Nucleic acid
- Polystyrene
- Protein
The monomer of polymer
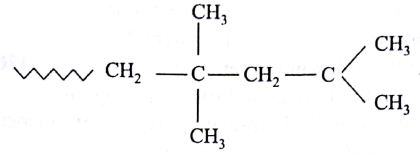
is
(AIPMT/NEET-2005)
-
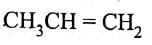
-
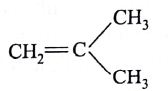
-
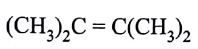
-
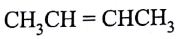
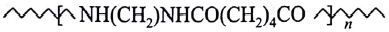
is a:
(AIPMT/NEET-2006)
- Thermosetting polymer
- Homopolymer
- Copolymer
- Addition polymer
Polymer obtained by condensation polymerization is:
(AIPMT/NEET-2007)
- Polythene
- Teflon
- PVC
- Nylon-6,6
Which one of the following statements is not true?
(AIPMT/NEET-2008)
- Natural rubber has the trans-configuration at every double bond
- Buna-S is a copolymer of butadiene and styrene
- Natural rubber is a 1, 4-polymer of isoprene
- In vulcanization, the formation of sulphur bridges between different chains make rubber harder and stronger
Structures of some common polymers are given. Which one is not correctly presented?
(AIPMT/NEET-2009)
-
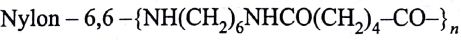
-
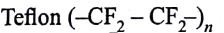
-
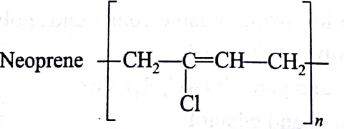
-
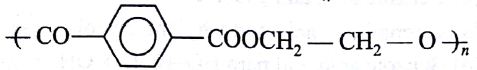
Which of the following represents neoprene polymer?
(AIPMT/NEET-2010)
-
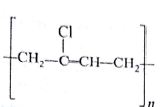
-
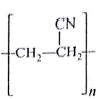
-
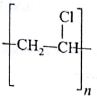
-
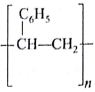
Of the following which one is classified as polyester polymer?
(AIPMT/NEET-2011)
- Nylon-6,6
- Bakelite
- Terylene
- Melamine
Which one of the following is not a condensation polymer?
(AIPMT/NNET-2012)
- Dacron
- Neoprene
- Melamine
- Glyptal
Which of the following statements is false?
(AIPMT/NEET-2012)
- The repeat unit in natural rubber is isoprene
- Both starch and cellulose are polymers of glucose
- Artificial silk is derived from cellulose
- Nylon-6,6 is an example of elastomer
Which one of the following sets forms the biodegradable polymer?
(AIPMT/NEET-2012)
-
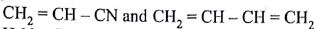
-
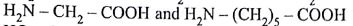
-
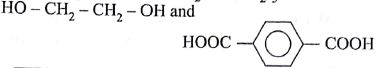
-

Which one of the following is an eample of thermosetting polymer?
(AIPMT/NEET-2014)
-
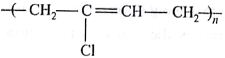
-
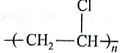
-
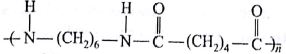
-
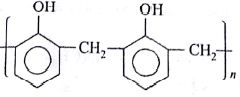
Which of the following organic compounds polymerizes to form the polyster Dacron?
(AIPMT/NEET-2014)
- Propylene and para
- Benzoic acid and ethanol
- Terephthalic acid and ethylene glycol
- Benzoic acid and para
Biodegradable polymer which can be produced from glycine and amminocaproic acid is
(AIPMT/NEET-2015)
- Buna-N
- Nylon-6,6
- Nylon 2-nylon 6
- PHBV
Caprolactum is used for the manufacture of :
(AIPMT/NEET-2015Re)
- Trylene
- Nylon-6,6
- Bylon-6
- Teflon
Natural rubber has :
(AIPMT/NEET-2016 Phase - I)
- Random cis- and trans-configuration
- All cis- configuration
- All trans-configuration
- Alternate cis- and trans- configuration
Which one among the following is a thermosetting plastic?
(AIIMS-2001)
- PVC
- PVA
- Bakelite
- Perspex
Which of the following polymer is an example of fibre?
(AIIMS-2002)
- Silk
- Dacron
- Nylon-66
- All of these
Which of the following is a biodegradable polymer?
(AIIMS-2003)
- Cellulose
- Polythene
- Polyvinyl chloride
- Nylon-6
Which of the following is not a natural polymer?
(AIIMS-2004)
- Cellulose
- Protein
- PVC
- Nucleic acid
Teflon is a polymer of the monomer or Teflon is obtained by the polymerisation of
(AIIMS-2005)
- Monofluoroethene
- Difluoroethene
- Trifluoroethene
- Tetrafluoroethene
The compound required for the formation of a thermosetting polymer with methanol is
(AIIMS-2006)
- Benzene
- Phenyl amine
- Benzaldehyde
- Phenol
Orlon is a polymer of
(AIIMS-2007)
- Styrene
- Tetrafluoro ethylene
- Vinyl chloride
- Acrylonitrile
Neoprene is a polymer of
(AIIMS-2009)
- Propene
- Vinyl chloride
- Chloroprene
- Butadiene
Which one of the following is used to make 'non-stick' cookware?
(AIIMS-2009)
- PVC
- Polystyrene
- Polyethylene terephthalate
- Polytetrafluoroethylene
Nylon-66 is
(AIIMS-2010)
- Polyamide
- Polyester
- Polystyrene
- Polyvinyl
In elastomer, intermolecular forces are
(AIIMS-2010)
- Nil
- Weak
- Strong
- Very strong
Perlon is
(AIIMS-2011)
- Rubber
- Nylon-6
- Terelene
- Oxlon
Which of the following is not polyamide?
(AIIMS-2012)
- Nylon-66
- Protein
- Glyptol
- Nylon-6
Which of the following statement is correct regarding the drawbacks of raw rubber?
(AIIMS-2015)
- It is plastic in nature
- It has little durability
- It has large water-absorption capacity
- All of these
is
is a: