Arrhenius Equation and Activation Energy
Arrhenius Equation
For a chemical reaction with
rise in temperature by 10°, the rate constant is nearly doubled.
The
temperature dependence of the rate of a chemical reaction is given by Arrhenius
equation,
![]()
where,
A = frequency or Arrhenius factor or pre-exponential factor,
R = gas constant and
Ea =
activation energy
Taking log on
both sides, we get,
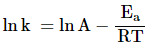
![]()
In the
Arrhenius equation, when T → ∞ or Ea =
0 then
k = Ae0 =
A,
and the rate
of reaction becomes independent
of temperature

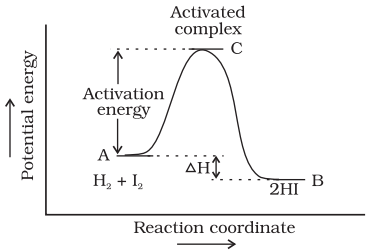
Activation Energy (Ea)
The additional amount of energy, required by the reactant so
that their energy (ER)
becomes equal to the threshold value is known as activation energy.
Ea = ET -
ER
Lower the
activation energy, faster is the reaction.
Different
reactions have different rates because their activation energies are different.
Larger the
value of Ea, smaller the
value of rate constant and greater is the effect of a given temperature rise on
k.
Activated complex (or transition state)
Activated complex is the highest energy unstable intermediate
between the reactants and products and gets decomposed immediately (having very
short life), to give the products. In this state, bonds of reactant are not
completely broken while the bonds of products are not completely formed.
Example
H2 (g) + I2 (g) → 2HI (g)