Adsorption
Adsorption:
The accumulation of molecular species at the surface
rather than in the bulk of a solid or liquid is termed adsorption.
·
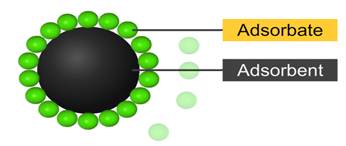
The molecular species or substance, which concentrates
or accumulates at the surface is termed adsorbate
·
The material on the surface of which the adsorption
takes place is called adsorbent
·
Adsorption
is essentially a surface phenomenon
Difference between Adsorption and
Absorption:
Adsorption:
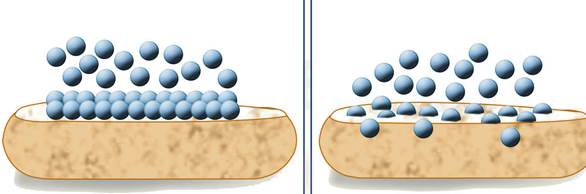
The substance is concentrated only at the surface and
does not penetrate through the surface to the bulk of the adsorbent
Absorption:
The substance is uniformly distributed throughout the
bulk of the solid
Adsorption and Absorption
|
Observation |
Absorption |
Adsorption |
|
Phenomenon |
Bulk phenomenon |
Surface phenomenon |
|
Heat
exchange |
Endothermic
process |
Exothermic
process |
|
Temperature |
It is not affected by temperature |
It is favoured by low temperature |
Mechanism of Adsorption:
·
Adsorption
arises due to the fact that the surface particles of the adsorbent are not in the
same environment as the particles inside the bulk.
·
The
extent of adsorption increases with the increase of surface area per unit mass
of the adsorbent at a given temperature and pressure.
·
Adsorption
is invariably an exothermic process
·
Adsorption
is thus accompanied by decrease in enthalpy as well as decrease in entropy of
the system
·
∆G
must be negative
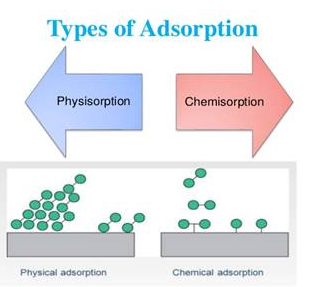
Types of Adsorption:
1. Physisorption
2. Chemisorption
Physisorption:
It is the accumulation of gas on the surface of a
solid which occurs on account of weak van der Waals’ forces.
·
A
physical adsorption at low temperature may pass into chemisorption as the
temperature is increased.
Example:
·
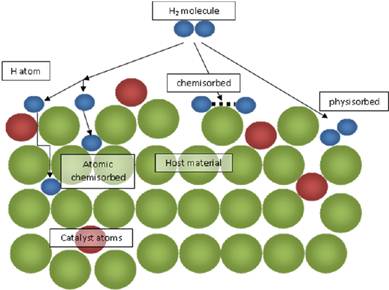
Di hydrogen is first adsorbed on nickel by van der
Waals’ forces.
·
Molecules
of hydrogen then dissociate to form hydrogen atoms which are held on the
surface by chemisorption.
Characteristics of Physisorption:
Lack of specificity:
·
A given surface of an adsorbent does not show any
preference for a particular gas
Reversible nature:
·
Physical adsorption of a gas by a solid is generally
reversible
Surface area of adsorbent:
·
The extent of adsorption increases with the increase
of surface area of the adsorben
Enthalpy of adsorption:
·
Enthalpy of chemisorption is high
Chemisorption:
Chemisorption is when
the gas molecules or atoms are held to the solid surface by chemical bonds.
·
The
chemical bonds may be covalent or ionic in nature.
·
Chemisorption
involves a high energy of activation
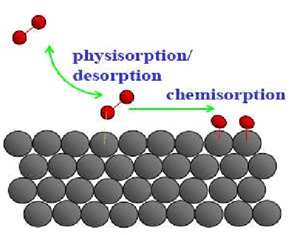
Characteristics of Chemisorption:
High Specificity:
·
Chemisorption is highly specific and it will only
occur if there is some possibility of chemical bonding between adsorbent and
adsorbate
Irreversibility:
·
Chemisorption
is also an exothermic process but the process is very slow at low temperatures
Surface Area:
·
chemisorption
also increases with increase of surface area of the adsorbent
Enthalpy of Adsorption:
·
Enthalpy
of chemisorption is high
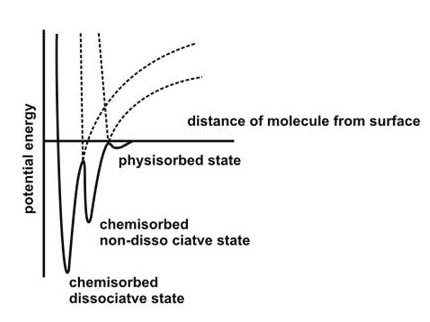
Comparison of Physisorption
and Chemisorption:
|
Physisorption |
Chemisorption |
|
Only
van der Waals force are present between adsorbate
and surface of adsorbent |
Chemical bonds are formed
between adsorbate and surface of adsorbent |
|
Low
enthalpy of adsorption ie, in the order of 20 kjmol-1. |
High enthalpy of adsorption i.e, order of 200 kjmol-1. |
|
Reversible |
Irreversible |
|
It usually
takes place at low temperature and does not require any activation
energy. |
It takes place at high
temperature and require activation energy. |
|
Multi
molecular layer of adsorbate are formed on the
surface |
Only monomolecular
layers are formed. |
|
Not
specific. |
Highly specific. |
Adsorption Isotherms:
Adsorption isotherm is the variation in the amount of
gas adsorbed by the adsorbent with pressure at constant temperature can be
expressed by means of a curve.
Freundlich adsorption isotherm:
·
Freundlich, in 1909, gave an
empirical relationship between the quantity of gas adsorbed by unit mass of
solid adsorbent and pressure at a particular temperature
![]() = k.p 1/ n
(n > 1)
= k.p 1/ n
(n > 1)
Adsorption from Solution Phase:
Solids can adsorb solutes from solutions also.
Example:
·
The precipitate of Mg (OH) 2 attains blue
colour when precipitated in presence of magneson
reagent.
·
The colour is due to adsorption of magneson.
Observation of Adsorption in Solution Phase:
·
The extent
of adsorption decreases with an increase in temperature
·
The
extent of adsorption increases with an increase of surface area of the
adsorbent
·
The
extent of adsorption depends on the concentration of the solute in solution
·
The
precise mechanism of adsorption from solution is not k
Application of Adsorption:
Production of High Vacuum:
The
remaining traces of air can be adsorbed by charcoal from a vessel evacuated by
a vacuum pump to give a very high vacuum.
Gas Masks:
Used
for breathing in coal mines to adsorb poisonous gases.
Control of Humidity:
Silica
and aluminium gels are used as adsorbents for removing moisture and controlling
humidity.
Separation of Inert Gases:
A
mixture of noble gases can be separated by adsorption on coconut charcoal at different
temperatures.
Froth Floatation Process:
A
low grade sulphide ore is concentrated by separating it from silica and other
earthy matter by this method using pine oil and frothing agent.
Chromatographic Analysis:
Chromatographic
analysis based on the phenomenon of adsorption finds a number of applications
in analytical and industrial fields.