Group 17 Elements
The 17 group of Periodic Table contains five elements
fluorine (F), chlorine (Cl), bromine (Br), iodine (I)
and astatine (At) collectively known as halogens (salt forming elements).
Astatine is artificially prepared radioactive element.
Occurrence
Being very reactive in nature, they are not found free
in nature. Their presence in earth’s crust follows the order.
F2 >
Cl2 >
Br2 >
I2
Fluorine is present mainly as
insoluble fluorides (fluorspar CaF2, cryolite Na3AlF6 and
fluoroapatite 3Ca3(PO4)2.CaF2) and
small quantities are present in soil, river water plants and bones and teeth of
animals.
Sea water contains chlorides, bromides and iodides of
sodium, potassium, magnesium and calcium, but is mainly sodium chloride
solution (2.5% by mass). The deposits of dried up seas contain these compounds,
e.g., sodium chloride and carnallite, KCl.MgCl2.6H2O.
Certain forms of marine life contain iodine in their systems; various seaweeds,
for example, contain upto 0.5% of iodine and Chile
saltpetre contains upto 0.2% of sodium iodate.
Electronic configuration
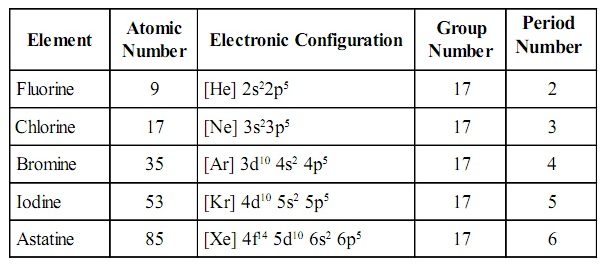
The valence shell electronic
configuration of these electrons is ns2np5. Thus, there are 7 electrons in the outermost shell of these
elements.