p- Block Elements
The last
electron in the electronic configuration enters the p-orbital of the p-block
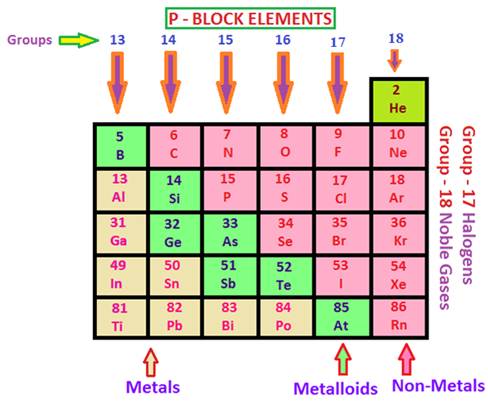
elements. Elements in Groups 13-18
of the Periodic Table are called P-block elements. These
include metals, metalloids, noble gases and halogens. Some of the commonly
known elements in the P-block are:
Ø Metals: Aluminium (Al), Boron
(B), Tin (Sn).
Ø Metalloids: Silicon (Si),
Germanium (Ge)
Ø Noble Gases: Helium
(He), Neon (Ne), Argon (Ar).
Ø Halogens: Fluorine (F), Chlorine (Cl), Bromine (Br).
Definition of p-Block
Elements
having a place within the group 13 (i.e. group IIIA) to group 17 (i.e.
group VIIA) of the periodic table alongside the group 18 i.e.
the zero group elements together frame the p-block of the periodic table.
Position of p-Block Elements in the Periodic Table
In the
elements of p-block, the last electron enters the furthest p orbital. They have
3 to 8 electrons in the peripheral shell. As we realize that the quantity of p
orbitals is three and, therefore, the most extreme number of electrons that can
be obliged in an arrangement of p orbitals is six. Consequently, there are six
groups of p-block elements in the periodic table numbering from 13 to
18.
Ø First group: group IIIA
called as Boron group
Ø Second group: group IVA
called as Carbon group.
Ø Third group: group VA
called as Nitrogen group.
Ø Fourth group: group VIA
called as Chalcogens.
Ø Fifth group: group VIIA
called as Halogens.
Ø Sixth group: zero group or
group 18 called as Inert or Noble gasses group.
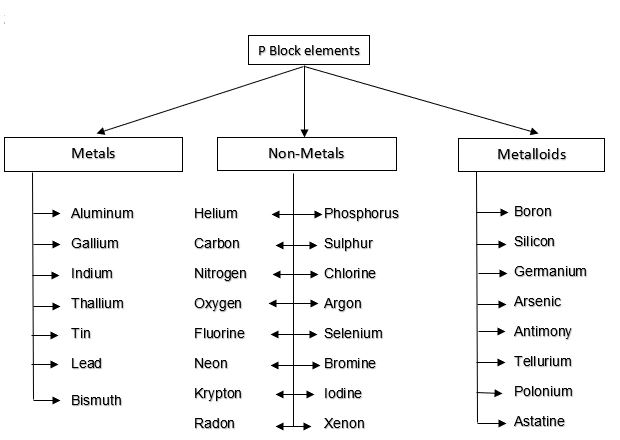
In the
p-block, all the three sorts of elements are available, i.e. the Metals, Non-Metals,
and Metalloids. The crisscross line in the p-block isolates every
one of the elements that are metals from those that are non-metals. Metals are
found on the left of the line, and non-metals are those on the right. Along the
line, we discover the metalloids. Because of the nearness of a wide range of
elements, the p-block demonstrates a great deal of variety in properties.