Electrostatics
Ø Electrostatics is
a branch of physics which deals with static electric charges. Since classical
physics, it has been known that some materials such as amber attract
lightweight particles after rubbing.
Ø The Greek word
for amber or electron, was
the source of the word 'electricity'.
Ø Electrostatic
phenomena arise from the forces that electric charges exert on each
other. Such electricity produced by friction is called as frictional
electricity.
Ø If
the charges in a body do not move, then the frictional electricity is also
known as Static Electricity.
Two kinds of charges
i.
If a glass is rubbed with a
silk cloth, glass acquires positive charge while the silk cloth acquires an
equal amount of negative charge.
ii.
If an ebonite rod is rubbed
with fur, it becomes negatively charged while the fur acquires equal amount of
positive charge.
These
classification of positive and negative charges were termed by American
scientist, Benjamin Franklin.
Like and Unlike charges-Experimental
verification:
A charged glass rod (can be positive or negative. Here in
figure glass is positively charged) is suspended by a silk thread such that it
swings horizontally. Now another charged glass rod of same charge is brought
near the other end of the suspended glass rod. It is found that the ends of the
two rods repel each other as shown in the figure.
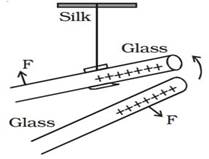
Two
charged rods of same sign
In
the figure, F is the force exerted by the glass rods to move away from one
another.
Now
if the glass rod is replaced with negatively charged ebonite rod, the two rods
(i.e. the glass rod and the ebonite rod) attract each other as shown in the
figure.
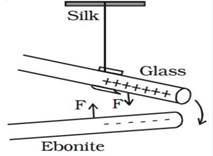
Two
charged rods of opposite sign
In
this figure, F is the force exerted by glass rod and ebonite rod to attract
each other.
The
property of attraction and repulsion between charged bodies have many
applications such as
Ø Electrostatic
paint spraying,
Ø Powder
coating,
Ø Fly-ash
collection in chimneys,
Ø Ink-jet
printing,
Ø Photostat
copying (Xerox) etc.
Conductors and Insulators
According
to the electrostatic behavior, materials are divided into two categories:
·
Conductors
·
Insulators (dielectrics)
Ø Bodies
which allow the charges to pass through are called conductors. Eg: Metals, human body, Earth etc.
Ø Bodies
which do not allow charges to pass through are called insulators. Eg: Glass, mica,
ebonite, plastics etc.
When some charge is transferred to the conductor, it readily
gets distributed over the entire body of the conductor. On a contrast when some
charge is transferred to an insulator, the insulator remains the same (i.e.
there is no transfer of charges in the non-conductor).
This property of material tells us why an electric comb gets
electrified when we comb a dry hair. This is not the case a metal body.
Charge on the metal leak through our body to the ground as
both are conducting materials. When we bring a charged body in contact with the
ground, the excess charges present in the body disappears by causing a
momentary current to pass through the ground connecting the conductor. This
process of sharing the electric charges with the ground is called as Earthing or Grounding. This prevent
humans from electrical shock when the appliances are grounded. A thick metal plate
is buried deep into the earth and thick wires are drawn from this plate; these
are used in buildings for the purpose of earthing
near the mains supply.
The
electric wiring in our houses has three wires: live, neutral and earth. The
first two carry electric current from the power station and the third is
earthed by connecting it to the buried metal plate. Metallic bodies of the
electric appliances such as electric iron, refrigerator, TV are connected to
the earth wire. When any fault occurs or live wire touches the metallic body,
the charge flows to the earth without damaging the appliance and without
causing any injury to the humans; this would have otherwise been unavoidable
since the human body is a conductor of electricity.

Charging by
Induction
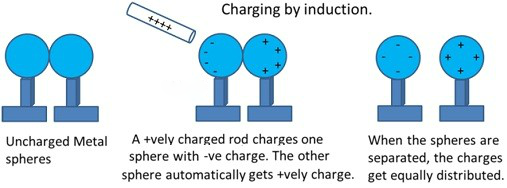
The above figure
explains clearly about the effect of charging by induction.
Consider
two metal spheres which are uncharged. Bring these bodies nearby which are
supported by an insulating stand.
Bring
a positively charged rod near any one of the uncharged sphere such that the rod
shouldn’t touch the other sphere. The free electrons present in the spheres are
attracted towards the rod. This leaves an excess of positive charge on the rear
surface of the other sphere. Both kinds of charges are bound in the metal
spheres and cannot escape. The left surface of the first sphere has an excess
of negative charge and the right side of the second sphere has an excess of
positive charge. However, not all of the electrons in the spheres have
accumulated on the left surface of the first sphere. As the negative charge
starts building up at the left surface of the first sphere, other electrons are
repelled by these. In a short time, equilibrium is reached under the action of
force of attraction of the rod and the force of repulsion due to the
accumulated charges. This process is called induction of charge and happens
almost instantly.
The
accumulated charges remain on the surface, till the glass rod is held near the
sphere. If the rod is removed, the charges are not acted by any outside force
and they redistribute to their original neutral state.
Even
if the spheres are kept at some distance when the positively charged rod is
present, the spheres are found to be oppositely charged and opposite to each
other.
When
the rod is removed and the spheres are separated, the charges in the spheres
are equally distributed within the spheres.
In the above
process the positively charged rod doesn’t lose its charges, contrary to the
process of charging by contact.
Properties of electric charges
The
properties of electric charges are
I.
Quantization of electric
charges,
II.
Conservation of electric
charges,
III.
Additive nature of charges.
All
these properties are explained below
I.
Quantization of electric charges:
The fundamental unit of electric charge is the charge carried
by the electron and its unit is Coulomb. The value of e is 1.6 x 10-19 C .
In nature, the electric charge of any system is always an
integral multiple of the least amount of charge. It means that the quantity can
take only one of the discrete set of values. The charge, q= ne.
Here
n is an integer and e is the elementary charge whose value
is mentioned above.
II.
Conservation of electric charges:
Electric
charges can neither be created nor destroyed.
According
to the law of conservation of electric charges, the total charge in an isolated
system always remains constant. But the charges can be transferred from one
part of the system to another, such that total charge always remains conserved.
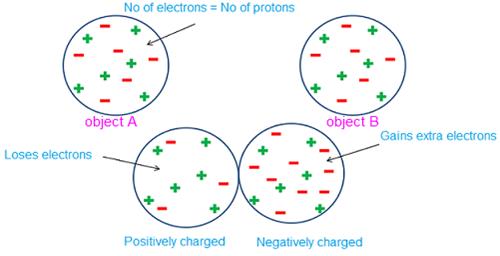
In the above diagram,
the negative charges from object A is transferred to the object B. Object A
loses electrons (negatively charged) and object B gains the electrons
(negatively charged). Hence the total number of negative charges are balanced.
Another
example: Uranium can decay by emitting alpha particles and get transformed to
thorium. It is given as
92U238 → 90Th234 + 2He4
Total
charge before decay = 92e whereas total charge after decay = 90e + 2e. Hence
the total charge is conserved i.e. it remains constant.
III.
Additive nature of charge:
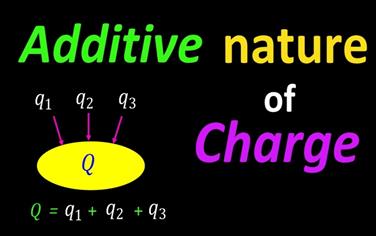
The
total electric charge of a system is equal to the algebraic sum of electric
charges located in the system. For example, if two charged bodies of charges
+2q, -5q are brought in contact, the total charge of the system is -3q (-5q +
2q = -3q ).