Radioactivity
The
spontaneous transformation of an element into another with the emission of some
particle (or particles) or electromagnetic radiation is called natural
radioactivity.
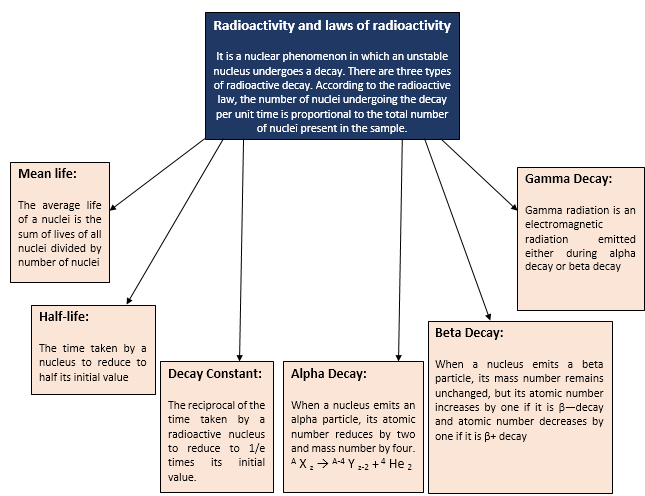
Laws of Radioactivity Decay
Rutherford
and Soddy studied the phenomenon of radioactivity in details and formulated the
following laws, known as the laws of radioactive decay:
1.
Radioactivity is a spontaneous phenomenon and one cannot predict,
when a particular atom in a given radioactive sample will undergo
disintegration.
2.
When a radioactive atom disintegrates, either an
D-particle (nucleus of helium) or a E-particle (electron) is emitted.
3.
The emission of an ![]() -particle
by a radioactive atom results in a daughter atom, whose atomic number is 2 units
less and mass number is 4 units less than that of the parent atom.
-particle
by a radioactive atom results in a daughter atom, whose atomic number is 2 units
less and mass number is 4 units less than that of the parent atom.
![]()
4.
The emission of a β-particle by a radioactive atom results ina daughter atom, whose atomic
number is 1 unit more but mass number is same as that of the parent atom.
![]()
5.
The number of atoms disintegrating per second of a radioactive
sample at any time is directly proportional to the number of atoms present at
that time. The rate of disintegration of the sample cannot be altered by
changing the external factors, such as pressure, temperature etc. It is known
as radioactive decay law.
According
to radioactive decay law, the rate of disintegration at any time t is directly
proportional to the number of atoms present at time t i.e.,
![]() or
or ![]() .
.
Where the constant of proportionally O is
called decay constant of the radioactive sample. It is also known as
disintegration constant or transformation constant. Its value depends upon the
nature of the radioactive sample. Further, the negative sign indicates that the
number of the atoms of the sample decreases with the passage of time.
From equation, we have ![]()
or ![]()
or ![]()
Radioactive Decay Constant
According to radioactive decay law,
integrating, we have
![]()
or, ![]()
Hence,
radioactive decay constant of a substance (radioactive) may be defined as the
ratio of its instantaneous rate of disintegration to the number of atoms present
at that time.
Again,
![]()
If ![]()
Then, ![]()
Hence,
radioactive decay constant of a substance may also be defined as the reciprocal
of the time, after which the number of atoms of a radioactive substance
decreases to 0.368 (or 36.8%) of their number present initially.
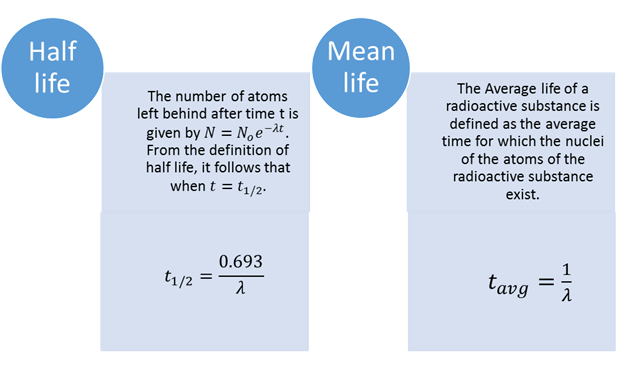
Half Life
Consider
that a radioactive sample contains ![]() atoms at time t = 0. Then, the number of atoms left behind after time t is given
by
atoms at time t = 0. Then, the number of atoms left behind after time t is given
by ![]()
From the definition of half-life, it follows
that when![]() ,
,
![]()
Setting the above condition in equation, we
have
![]()
or, ![]()
or, ![]()
or, ![]()
Thus, half-life of a radioactive substance is
inversely proportional to its decay constant and is characteristic property of
its nucleus. It cannot be altered by any known method.
Mean Life or Average Life
The Average life of a radioactive substance is
defined as the average time for which the nuclei of the atoms of the
radioactive substance exist. It is defined by ![]()
![]()
Activity of radioactive substance
The
activity of a radioactive substance may be defined as the rate at which the
nuclei of its atoms in the sample disintegrate.
If a
radioactive sample contains N atoms
at any time t, then its activity at
time t is defined as ![]()
The negative sign shows that with the passage
of time, the activity of the radioactive substance decreases.
Since according to the radioactive decay law,
![]()
The equation may be expressed as, ![]() . Since,
. Since,![]() we have
we have
![]()
![]()
Here, ![]()
![]() is activity of the radioactive
sample at time t = 0.
is activity of the radioactive
sample at time t = 0.
Units of Activity
The activity
of a radioactive sample may be expressed as disintegration per second. The
practical unit of activity of a radioactive sample is curie (ci).
The
activity of a radioactive sample is called one curie, if it undergoes ![]() disintegrations per second. Thus,
disintegrations per second. Thus,
1 curie (ci) = ![]() disintegrations
disintegrations ![]()
There is also another unit of radioactivity,
called Rutherford (rd).
The activity of a radioactive sample is called
one Rutherford, if it undergoes ![]() disintegration per second.
disintegration per second.
1 Rutherford (rd) ![]() disintegration
disintegration![]() .
.