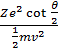Rutherford's
α-Scattering Experiment
 s
s
Thomson's Atomic Model:
According to this model, an atom consists of a
positively charged sphere in which entire mass & positive charge of the
atom is uniformly distributed. Inside this sphere, the electrons are embedded
like seeds in a watermelon or like plums in a pudding. The number of electrons
is such that their negative charge is equal to positive charge. Thus, atoms is
electrically neutral.
Limitations of Thomson's Model:
i.
Could
not explain the origin of spectral series of hydrogen & other atoms.
ii.
Could
not explain large angle scattering of α - particles observed by
Rutherford.
Rutherford's α-scattering
experiment:
An α - particle is He nucleus containing 2
protons & 2 neutrons. It has 4 units of mass & 2 units of positive
charge. Many radioactive elements emit α - particles.
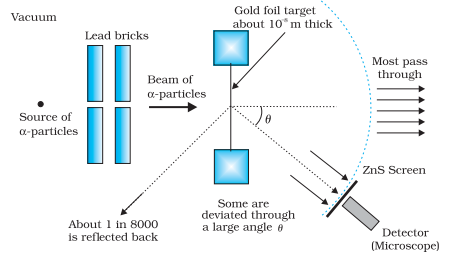
S is a radioactive source contained in a lead
cavity. The α - particle emitted by the source are collimated into narrow
beam with the help of collimator. The collimated beam is allowed to fall on a
thin gold fail of thickness ≈ 10–5 m. α - particles are
scattered in different directions are observed though a rotatable detector
consisting of ZnS screen & a microscope. The α – particles produce
bright flashes on ZnS screen. These are observed by the microscope &counted
at different scattering angle θ.
Observations:
i.
Most
of the α - particle pass straight through the gold foil or suffered very
small angle of deflections.
ii.
A
few a- particles scatter through large ogles ( > 90°).
iii.
Rarely,
an α - particle rebounces i.e., scattered through an angle of 180°
Explanation:
i.
Since
most of the α - particles passed undeviated, the atom has a lot of empty
space in it.
ii.
To
explain large scattering of α - particles, Rutherford suggested that all
the positive charge & entire mass of the atom is confined to an extremely
small central core called as nucleus.
iii.
The
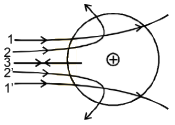
scattering of α - particles through different angles was explained as :
The α - particles I, I’. Which pass through the atom at a large
distance from from the nucleus experience a small electrostatic force of
repulsion & undergo a small defection. The α - particles 2. 2’ which
pass through the atom at a close distance from the nucleus suffer a larger
defection. The α – particle 3, which travels directly towards the nucleus
shows down, comes to rest & is deflected through 180° and hence retraces
its path.
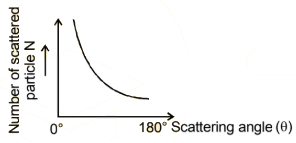
The graph between scattering angle θ & the number of α -
particles directly scatted N is as shown:
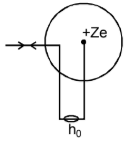
Distance of closet Approach:
An α - particle travelling towards the
center of the nucleus slows down as it approaches the nucleus. At a certain
distance, say r0 from the
nucleus, the α -particle comes to rest for a moment and then retraces it
path. It initial kinetic energy is completely converted into electrostatic
potential energy. This distance r0
is called the distance of closest approach. This distance gives an estimate of
the size of the nucleus.
Mathematically,
![]() mv2 =
mv2 = ![]()
![]()
where 2e is the charge on
α - particle and Ze is the charge on nucleus.
![]() =
=
![]()
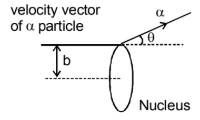
Impact Parameter:
Impact parameter is defined as the
perpendicular distance of the velocity vector of the α - particle from the
center of the nucleus, when it is far away from the nucleus of the atom.
Rutherford derived the relation between impact parameter and scattering angle,
which is given by
b = ![]()