Periodic
Classification of Elements
“The periodic table is a tabular method of
displaying the elements in such a way, that the elements having similar
properties occur in the same vertical column or group”.
Earlier attempts of the classification of elements:
Dobereiner’s Triads, Newland’s law of octaves.
Dobereiner’s Triads:

This classification is based on the atomic mass.
According to this, when elements are arranged in order of increasing atomic
masses, groups of three elements, having similar properties are obtained. The
atomic mass of middle element of the triad being nearly equal to the average of
the atomic masses of the other two elements.
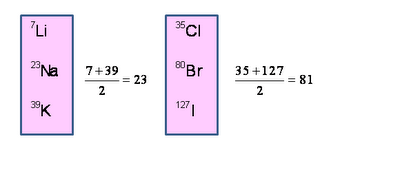
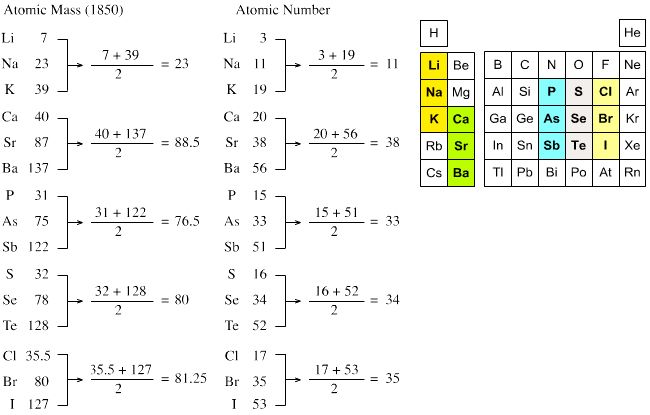
For Example Li (6.9), Na (23), K (39).
Example 1-
|
Li |
Na |
K |
7 23
39
Alkali gp.
These elements have some chemical
properties as follows -
i.all are
metals
ii.all react
with water to form alkalis
iii.all have
valency-1
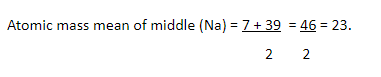
Example 2-
|
Ca |
Sr |
Ba |
40 88
137 Alkaline gp.
These elements have some chemical
properties as follows -
i.all are
metals
ii.oxides of
then are alkaline
iii.all have valency 2.
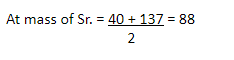
Example 3 -
|
Cl |
Br |
I |
35.5 80
127 halogen gp.
These elements have some chemical
properties as follows -
i.All are
non-metals
ii.All react
with water of form acids
iii.All have valency 1
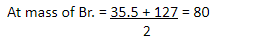
Limitation: It fails to arrange all
the known elements in the form of triads, even having similar properties.
Newland’s Law of Octaves:
According to this ‘when elements are placed in
order of increasing atomic masses, the physical and chemical properties of
every 8th element are a repetition of the properties of the first element.’
Form of Newland’s octaves is given in the following
table:
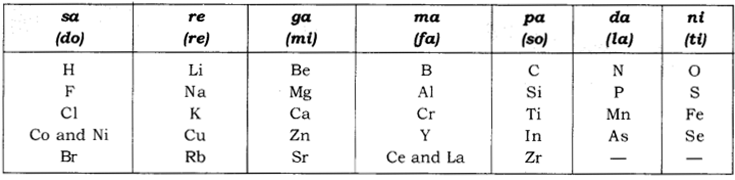
Limitations
·
Law of octaves was applicable only upto calcium (only for lighter elements).
·
Newland adjusted two elements in the same slot
(e.g. Co and Ni), having different properties. For example; Co and Ni with
Fluorine, Chlorine, Bromine and Iodine.
·
According to Newland, only 56 elements existed in
nature and no more elements would be discovered in future.
Present attempts for the classification of
elements: Mendeleev’s Periodic Table, the Modern Periodic Table.
Characteristics of Newlands’ Law of
Octaves:
·
It contained the elements from hydrogen to thorium.
·
Properties of every eighth element were similar to
that of the first element.
Table showing Newlands’ Octaves:
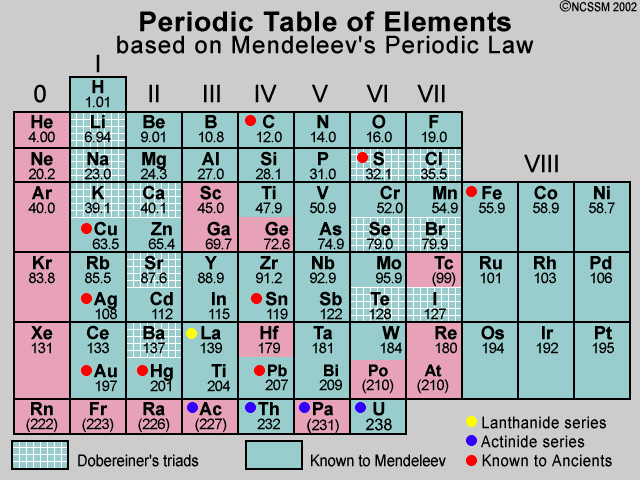
Mendeleev’s Periodic Table:
Mendeleev’s periodic table is based on the physical
and chemical properties of elements and their atomic masses.
Mendeleev’s Periodic Law:
According to this “The physical and chemical
properties of the elements are the periodic function of their atomic masses.”
Periodicity of Properties:
The repetition of properties of elements after
certain regular intervals is known as Periodicity of Properties.
Merits of
Mendeleev’s Periodic Table
·
Mendeleev’s left vacant places in his table which
provided an idea for the discovery of new elements. Example: Eka-boron, Eka-aluminium and Eka-silicon.
·
Mendeleev’s periodic table was predicted properties
of several undiscovered elements on the basis of their position in Mendeleev’s
periodic table.
·
It is useful in correcting the doubtful atomic
masses of some elements.
·
Noble gases could accommodate in the Mendeleev’s
periodic table without disturbing the periodic table after discovery.
Limitations
of Mendeleev’s Periodic Table
(a) No fixed position for hydrogen: No correct position of the hydrogen atom
was in Mendeleev’s periodic table.
Example: Position of hydrogen with alkali metals and halogens (17th group).
(b) No place for isotopes: Position of isotopes
were not decided.
Example: Cl-35 and Cl-37.
(c) No regular trend in atomic mass: Position of
some elements with lower atomic masses before with higher atomic mass.
Example: Ni-58.7 before Co-58.9.
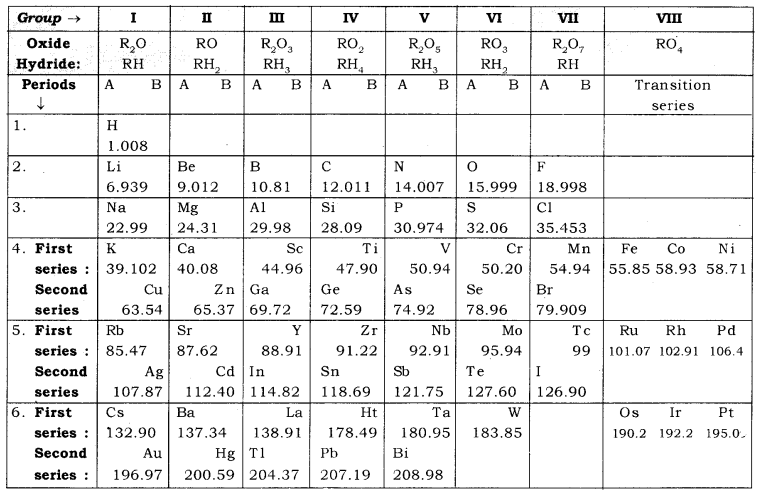
Mendeleev’s
original periodic table is reproduced in the table below
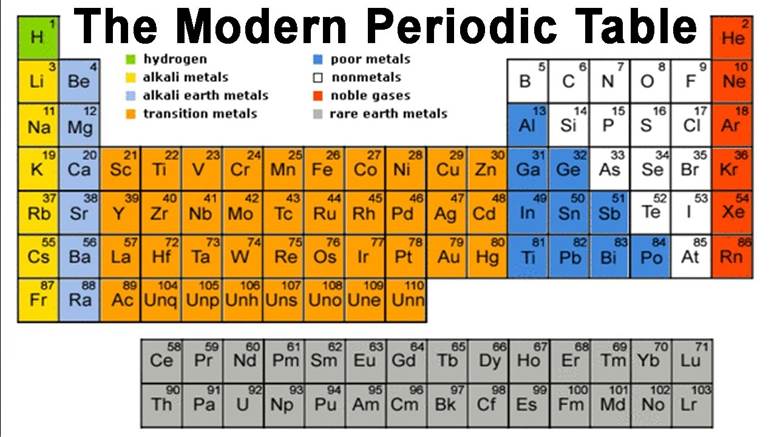
The Modern Periodic Table:
In 1913, Henry Moseley showed that the atomic
number of an element is a more fundamental property than its atomic mass.
Modern Period Law:
The physical and chemical properties of elements
are the periodic function of their atomic number.
Modern periodic table is based on atomic number of elements.
Atomic number (Z) is equal to the number of protons present in the nucleus of
an atom of an element.
Modern periodic table contains 18 vertical column known as group and seven
horizontal rows known as periods.
On moving from left to right in a period, the number of valence electrons
increases from 1 to 8 in the elements present.
On moving from left to right in a period, number of shell remains same.
All the elements of a group of the periodic table have the same number of
valence electrons.
Trends in Modern Periodic Table:
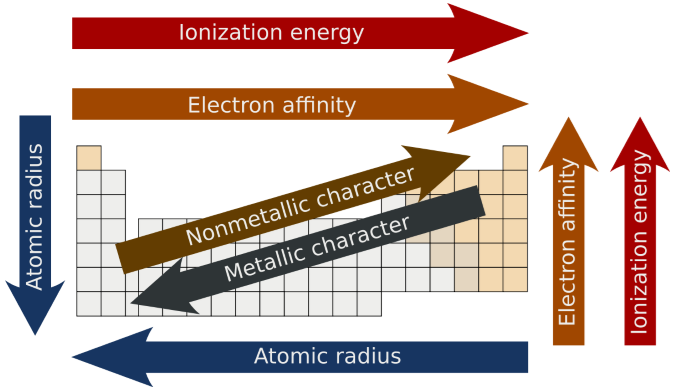
Valency, Atomic size, metallic
and non-metallic characters, and Electronegativity.
(i) Valency:
The valency of an element
is determined by the number of valence electrons present in the outermost shell
of its atom (i.e. the combining capacity of an element is known as its valency).
In Period: On moving from left to
right in a period, the valency first increases from 1
to 4 and then decreases to zero (0).

In Groups: On moving from top to
bottom in a group, the valency remains same because
the number of valence electrons remains the same.
Example: Valency of first group elements = 1 Valency of second group elements = 2.
(ii) Atomic size:
Atomic size refers to radius of an atom. It is a
distance between the centre of the nucleus and the outermost shell of an
isolated atom.
In Period : On moving from left to right in a period,
atomic size decreases because nuclear charge increases.
Example: Size of second period elements: Li > Be > B > C > N > O
> F
Point to know: The atomic size of noble gases in corresponding period is
largest
due to presence of fully filled electronic configuration (i.e. complete octet).
In Group: Atomic size increases down the group because new shells are being
added in spite of the increase in nuclear charge.
Example ; Atomic size of first group element : Li <
Na < K < Rb < Cs < Fr
Atomic size of 17th group elements : F < Cl < Br < I
(iii)
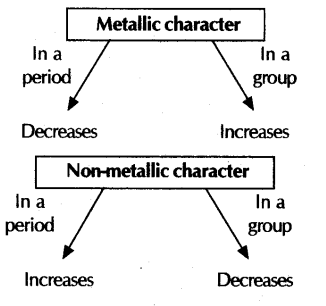
Metallic character:
It is the tendency of an atom to lose electrons. In
Period: Along the period from left to right, metallic characters decreases
because a tendency to lose electron decreases due to the increase in nuclear
charge. Example: Metallic character of second period elements: Li > Be >
B > C >> N > O > F
In Group: Metallic character, when moving from top to bottom increases because
the atomic size and tendency to lose electrons increases.
Example: First group element : Li < Na < K < Rb < Cs
17th group elements: F < Cl < Br < I
(iv)
Non-metallic character:
It is tendency of an atom to gain electrons.
In Period: Along the period from left to right, non-metallic character
increases because tendency to gain electrons increases due to increase in
nucleus charge. Example ; Non-metallic character of
2nd period elements : Li < Be < B < C < N < O < F In Group:
On moving from top to bottom in a group, non-metallic character decreases
because atomic size increases and tendency to gain electrons decreases. Ex.
Non-metallic character of 17th period element: F > Cl > Br > I
(v) Chemical Reactivity
In metals: Chemical reactivity of metals increases down the group because
tendency to lose electrons increases. Example ; Li
< Na < K < Rb < Cs (1st group) In
non-metals: Chemical reactivity of non-metals decreases down the group because
tendency to gain electrons decreases. Example: F > Cl > Br > I (17th
group)
(vi)
Electronegativity:
It is tendency of an element to attract the shared
pair of electrons towards it in a covalently bonded molecule. It increases with
increase of nuclear charge or decrease in atomic size.
Along the period electronegativity increases. Example ;Li
< Be < B < C < N < O < F. Down the group electronegativity
decreases. Example ; Li > Na > K > Rb > Cs
F > Cl > Br > I
(vii) Nature of Oxides:
Metal oxides are basic in nature. Ex. Na2O, MgO etc.
Non-metal oxides are acidic in nature. Ex. Cl2O7, SO3, P2O5,
In the case of metal reactivity, it increases down
the group because of the tendency to lose electrons increases.
In the case of non-metal reactivity, decreases down
the group because of the tendency to gain electrons decreases.
Group: The vertical columns in
Mendeleev’s, as well as in Modern Periodic Table, are called groups.
Period: The horizontal rows in
the Modern Periodic Table and Mendeleev’s Periodic Table are called periods.
There are 18 groups and 7 (seven) periods in the
Modern Periodic Table.
Atomic size: The atomic size may be
visualised as the distance between the centre of the nucleus and the outermost
shell of an isolated atom.
The trend of atomic size (radius) in
moving down a group:
Ongoing down in a group of the Periodic Table, the
atomic size increases because a new shell of electrons is added to the atoms at
every step. There is an increase in distance between the outermost shell
electrons and the nucleus of the atom.
The trend of atomic size (radius) in
moving from left to right in a period:
On moving from left to right along a period, the
size of atoms decreases because on moving from left to right, the atomic number
of elements increases which means that the number of protons and electrons in
the atoms increases. Due to the large positive charge on the nucleus, the
electrons are pulled in more closely to the nucleus and the size of the atom
decreases.
Characteristics of triads of J.W. Dobereiner.
·
Elements of a triad show similar chemical
properties.
·
These elements of a triad show specific trends in
their physical properties.
·
The atomic mass of the middle element was roughly
the average of the atomic masses of the other two elements.
Example: Atomic mass of Na is 23 in the triad Li,
Na and K. This atomic mass is the average of the atomic masses of Li and K
which have atomic masses 7 and 39 respectively.
Triads as formed by Dobereiner.
1st Triad
Li – Lithium
Na – Sodium
K – Potassium
2nd Triad
Ca – Calcium
Sr – Strontium
Ba – Barium
3rd Triad
Cl – Chlorine
Br – Bromine
I – Iodine
Mendeleev’s Periodic Law:
It states that “the properties of elements are the
periodic functions of their atomic masses.” It means the properties of the
elements depend on their atomic masses and the elements are given a position in
the periodic table on the basis of their increasing atomic masses.

Merits of Mendeleev’s Periodic Table
(i)
Mendeleev left a number of gaps in his table to accommodate the new elements
which would be discovered later on. So Mendeleev boldly predicted the existence
of some more elements. He even predicted the properties of some of these
elements and named them as Eka-boron, Eka-aluminium and Eka-silicon
respectively. Later on the elements were discovered, for example, gallium
replaced Eka-aluminium and it showed properties
similar to that of aluminium.
(ii) He gave the proper position to the noble gases
which were discovered later on, without disturbing the existing order of
elements. He placed them in a new group.
Limitations of Mendeleev’s
classification:
·
The position of isotopes could not be explained
because isotopes have the same chemical properties but different atomic masses.
If the elements are arranged according to atomic masses, the isotopes should be
placed in different groups of the Periodic Table.
·
The atomic masses do not increase in a regular
manner in going from one element to the next.
·
He could not assign a correct position to hydrogen
in his table because hydrogen has some properties similar to alkali metals and
some properties similar to halogens.