Work, Power And Energy
Work
The definition of work done may vary in real life
and scientifically. For Example, We may consider studying, talking,
singing, reading as work but it is not so in the case of science.
Examples of Scientific Work Done are:
·
Moving a chair from one location to another
·
Lifting a book from the shelf and placing it
on a table
·
Pushing a pebble lying on the ground.
In all these situations we are applying a force on
an object which is then changing the state of rest or motion of the object.
So, we can conclude that work is done if and only
if:
·
A force is applied to an object.
·
If the object is displaced from one point to
another point.
These are also called ‘Conditions of Work
Done’.
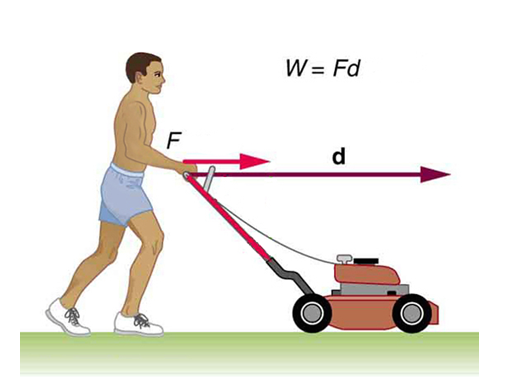
When you play a certain force ‘F Newton’ on an
object and the object moves a distance of ‘ d meters’ in the direction where
you applied the force then, the amount of work done can be calculated as:
|
Work
done = Force * Displacement W =
F * d |
Definition of Work Done: Work is defined as the
product of the force applied on an object and displacement caused due to the
applied force in the direction of the force. Work is a scalar quantity. It has
no direction of its own but a magnitude.
|
SI
unit of Work: N-m or J (Joule) |
What is 1 Joule Work?
A situation where 1 Newton force is applied on an
object that can move the object by a distance of 1m in the direction of the
applied force, then 1 joule of work is said to be done.
·
Depending upon the direction of displacement
and force applied the nature of work done may vary. Consider the table given
below:
|
The
direction of Displacement |
Work
Done |
Nature
of Work Done |
Angle
between Force applied and Displacement occurred |
|
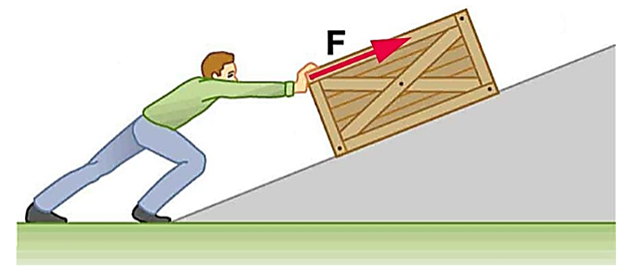
|
Same
as the direction of Force |
W =
F * d |
Positive |
0o
(Force and Displacement are Parallel to each other) |
|
|
Opposite
as direction of Force |
W =
-F * d |
Negative |
180o |
|
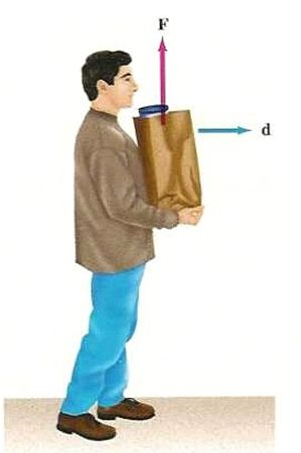
|
No
change in position |
W =
F * 0 = 0 |
Zero |
90o |
|
|
Positive Work Done |
|
|||
|
Negative Work Done |
|
|||
|
Zero Work Done |
|
|||
Energy
Any object that is capable of doing work processes
some energy. The object can gain or lose energy depending upon the work done.
If an object does some work it loses its energy and if some work is done on an
object it gains energy.
Different forms of energies
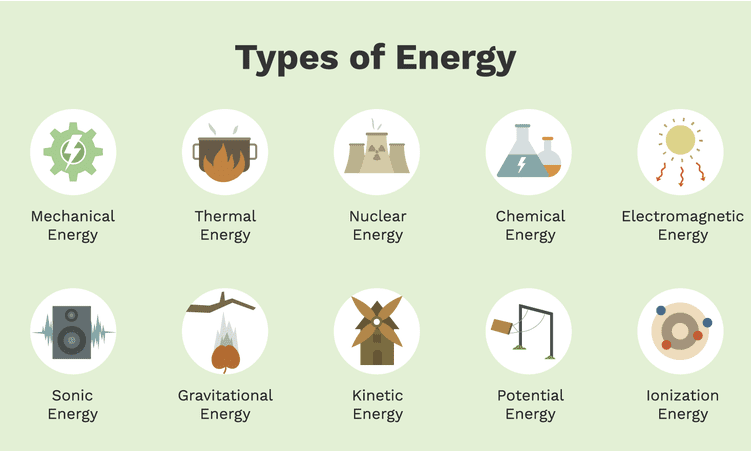
Kinetic Energy
Every moving object possesses some energy
called Kinetic Energy. As the speed of the object increases so is
its kinetic energy.
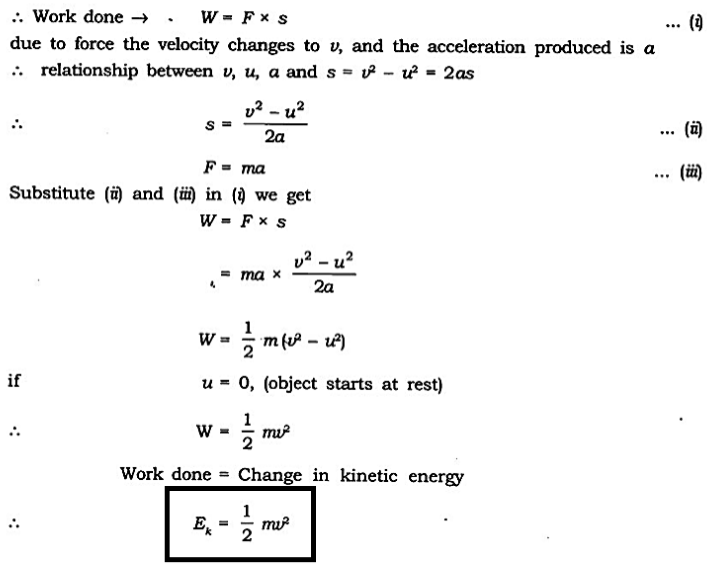
Formula for Kinetic Energy
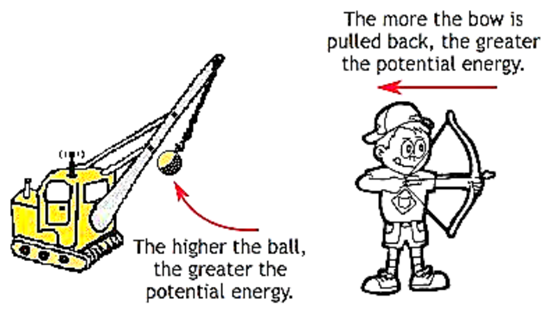
Potential Energy
Every object possesses some energy called Potential
Energy. An object when gains energy may store it in itself as potential
energy.
We know that when an object rises above the ground
some work is done against gravity. Since work is done on the object, the object
would gain some energy. The energy that the object gains at a height is
called Gravitational Potential Energy. It is defined as the amount
of work done required in raising an object above the ground up to a certain
point against the gravity
Consider the example given below,
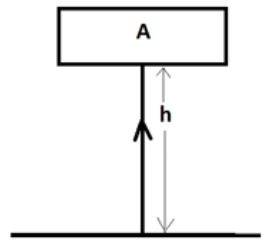
An object ‘A’ having mass ‘m’ is raised by height
‘h’ above the ground. Let us calculate the potential energy of object A at
height ‘h’:
We know that,
|
W =
F * d = F * h (height) And
F = m * g (because the force is applied against gravity) So,
W = m * g * h Hence
potential energy of object A, Ep = m * g * h |
·
Gravitational potential energy does
not get affected due to the path taken by the object to reach a certain height.
Other forms of Energies:
·
Mechanical Energy – It is the sum of
kinetic and potential energy of an object. Therefore, it is the energy obtained
by an object due to motion or by the virtue of its location. Example,
a bicycle climbing a hill possesses kinetic energy as well as potential energy.
·
Heat Energy – It is the energy obtained by an
object due to its temperature. It is also called Thermal Energy. Example,
energy possessed by a hot cup.
·
Chemical Energy – It is the energy
accumulated in the bonds of chemical compounds. Chemical energy is released at
the time of chemical reactions. Example, energy possessed by
natural gas and biomass.
·
Electrical Energy – It is kind of
kinetic energy caused due to the motion of electrons. It depends upon the speed
of electrons. As the speed increases so does the electrical energy. Example,
electricity produced by a battery, lightning at thunderstorms
·
Light Energy – It is the energy due
to light or electromagnetic waves. It is also called as Radiant Energy or Electromagnetic
Energy. Example, energy from the sun
·
Nuclear Energy – It is the energy
present in the nucleus of an atom. Nuclear energy releases when the nucleus
combines or separate. Therefore, we can say that every atom in this universe
comprises of nucleus energy. Example, uranium is a radioactive
metal capable of producing nuclear energy in nuclear power plants
·
Sonic Energy – It is the energy
produced by a substance as it vibrates. This energy flows through the substance
in the form of sound waves. Example, music instruments produce
sound energy
·
Ionization Energy – It is the energy
that binds electrons with its nucleus. It is thus the amount of energy required
to remove one electron completely from its atom (called First
Ionization Energy). Subsequently, the ionization energy increases as we
remove the second electron from the atom ( called Second
Ionization Energy).
- One form of energy can be transformed
into other forms of energy.
Law of Conservation of Energy
According to the law of conservation of energy, the
total amount of energy before and after transformation remains the same.
Consider the following example where an object of
mass ‘m’ is made to fall freely from a height ‘h’.
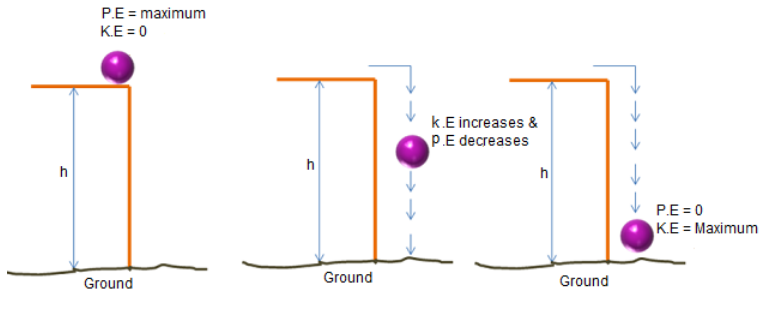
|
Instant |
Height at an instant |
Kinetic Energy |
Potential Energy |
Sum of KE + PE = ME |
|
1 |
Height = h |
0 (velocity is 0) |
mgh |
0 + mgh |
|
2 |
Height = k |
(1/2) mv12 (velocity = v1) |
mgk |
(1/2) mv12 + mgk |
|
3 |
Height = 0 |
(1/2) mv22 (velocity = v2) |
0 |
(1/2) mv22 + 0 |
We can see that the sum of kinetic energy and
potential energy at every instant is constant. Hence, we can say the energy
is conserved during transformation.
Power – The rate of doing work
is defined as Power.
|
Power
= Work Done / Time P =
W/ t SI
Unit: W (Watt) or J/s 1
kilowatt = 1000 watts 1 kW
= 1000 W 1 W
= 1000 J s-1 Average
Power = Total Energy Consumed / Total Time taken |
Commercial Unit of Power
We cannot use Joule to measure power commercially.
Instead, we use kilowatt-hour (kWh).
Commercial unit of energy = 1 kilowatt hour (kwh)
∴ 1 kWh = 1 kilowatt × 1 hour
= 1000 watt × 3600 seconds
= 3600000 Joule (watt × second)
1 kWh = 3.6 × 106 J.
∴ 1 kWh = 1 unit
The energy used in one hour at the rate of 1 kW is
called 1 kWh.