Natural Resources
Resource A Resource is a
stock or supply of something useful. Resources can be manmade or Natural.
Natural Resources A Resource which
exists in nature is called a Natural Resource. It is not produced
by any human being; rather it is available in nature itself. Human beings and
other animals depend upon these resources for their existence.
Natural Resources present on the
earth are:
·
Air The atmosphere which
contains different gases such as Oxygen, Nitrogen and carbon dioxide which are
required for the survival of life on earth.
·
Water The hydrosphere which
covers almost 75% of the Earth surface. It is a home to an abundance of animals
and plants and is also required for the survival of life on earth.
·
Land The upper crust of the earth is
called Lithosphere where different kinds of soils are found
which are necessary for the growth of plants and are a home to several vitamins
and minerals.
Life on Earth is possible because of the presence
of these three major sources. A zone where the lithosphere, the atmosphere and
the hydrosphere intersect and the life sustains is called the Biosphere.
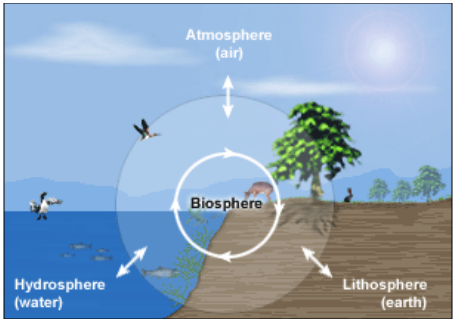
Figure 1 Natural Resources and Biosphere
The biosphere can be divided into two parts:
·
Abiotic Components The non-living
things such as air, water and land
·
Biotic Components The living
things such as plants and animals
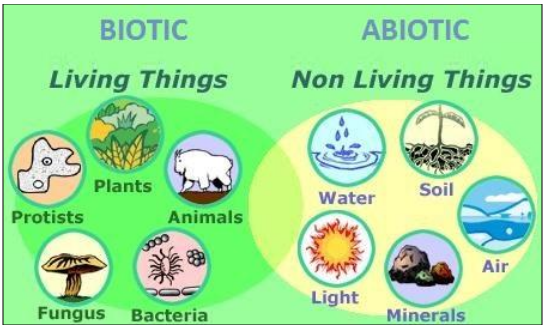
Figure 2 Biotic and Abiotic Components
Air
The air or the atmosphere comprises of various
gases. Life is present on the earth because of these gases especially oxygen.
Other planets such as Mars and Venus contain majorly carbon dioxide (95% to
97%) that is why life cannot exist there.
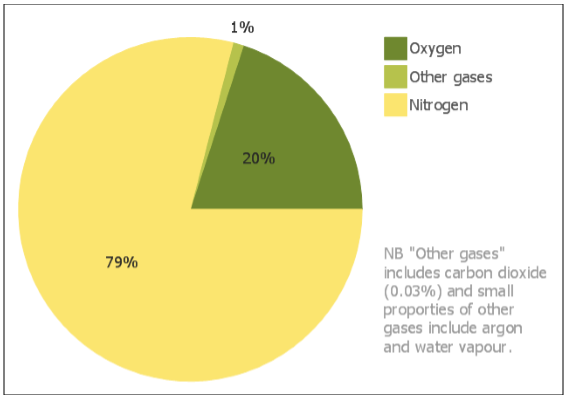
Figure 3 Composition of Air
How much carbon dioxide is produced
on earth?
·
The Eukaryotic cells and some prokaryotic
cells generate energy by breaking down glucose molecules. This results in the
production of carbon dioxide.
·
The process of combustion also leads to the
release of carbon dioxide in the atmosphere. How the amount of carbon dioxide
is regulated on the earth.
·
The plants present on the earth utilize
carbon dioxide present in the atmosphere and release oxygen as a byproduct in the process of photosynthesis.
·
The Marine animals make their shells in water
using carbonates dissolved in water
The atmosphere of the earth helps in
maintaining its temperature
·
The presence of gases around the earth
prevents an increase in the temperature of the earth during the daytime.
·
The atmosphere prevents the harmful
radiations of the sun from reaching the Earth's surface.
·
The atmosphere prevents the heat of the earth
in escaping the earths surface during the night time.
·
Moon has no atmosphere hence it faces extreme
temperatures ranging from -190 degrees to 110 degrees.
Winds
·
The changes in the earth's atmosphere are
responsible for Winds, Storms, Breezes and Rains.
·
The formation of water vapour in the
atmosphere can take place due to the heating of water bodies and activities of
living organisms.
·
The atmosphere can heat up as the earth,
water bodies and land, radiate back the heat they absorbed. The land gets
heated up or cooled down faster than water.
·
As the air gets heated, it rises above which
leads to winds.
Winds in the coastal regions
During the daytime, the land in the coastal areas
gets heated faster than the sea. As a result, the air above the land rises
above and creates a region of low pressure. Then, the air above the sea moves
to this reason of low pressure. This creates winds in the coastal area during
the day.
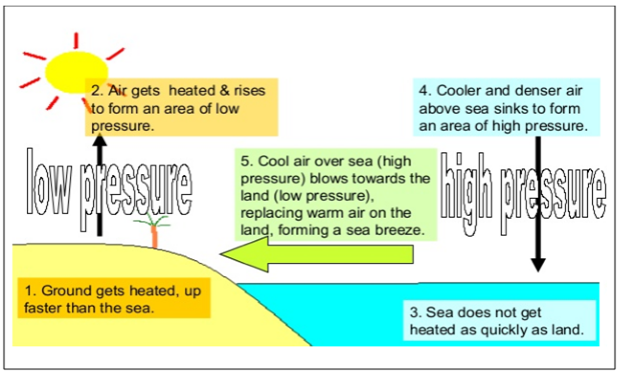
Figure 4 Winds in the coastal regions during Day
During the night time, the land cools down faster
than the sea. The air above the sea being warmer rises above and creates an
area of low pressure over the sea. As a result, the air above the land moves
towards the sea. This causes winds at the night time in the coastal regions.
(NTSE)
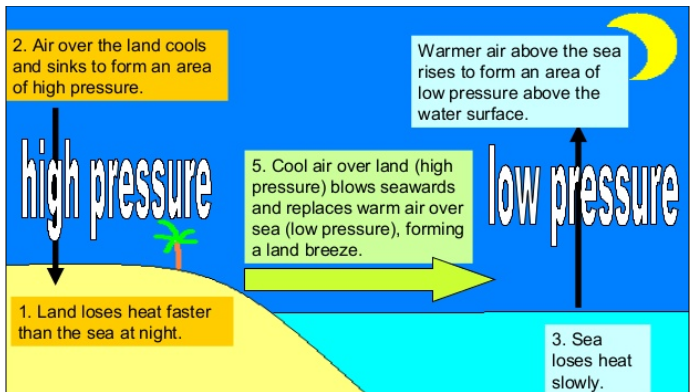
Figure 5 Winds in the coastal regions during Night
Other factors that also affect the formation of
winds
·
Uneven heating of the Earth's atmosphere
·
The rotation of the earth
·
The presence of mountains on the earth
The formation of Clouds
·
As the water bodies on the earth get heated
up water vapour is formed which is carried out in the atmosphere through hot
air.
·
When the air rises it expands and cools down.
The water vapour present in the air also cools down and forms tiny water
droplets.
·
There are other particles such as dust
present in the air. These particles act as a nucleus to these droplets.
·
The droplets condensate further and form
large droplets and thereby the clouds. Once they become very heavy, they fall
off on to the ground as rain.
·
If the temperature of the air is very low it
results in snowfall at some places.
·
The winds flowing in an area also affect the
rainfall of that place.
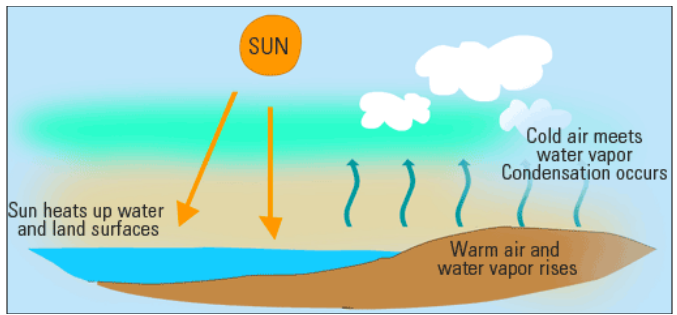
Figure 6 The formation of
Clouds
Air Pollution
The presence of harmful substances in the air leads
to pollution of the air. It can severely affect the health of living organisms
and the quality of the environment.
What causes Air Pollution?
·
Burning of Fossil Fuels When coal and
petroleum are burnt they release sulphur and nitrogen oxides which are harmful
agents. They also release unburnt carbon particles in the air called Hydrocarbons.
·
Exhaust from Industries The industries release
harmful gases and smoke in the air that contains carbon monoxide and organic
compounds that decrease the quality of the air.
·
Mining During the mining
process harmful chemicals are released in the air that leads to air pollution.
·
Indoor Activities Cleaning agents and
paints used in houses release harmful chemicals which pollutes the air
·
Suspended Particulate Matter The particles such
as dust often remain suspended in the air and degrade its quality. SPM is one
of the major causes of air pollution in the cities.
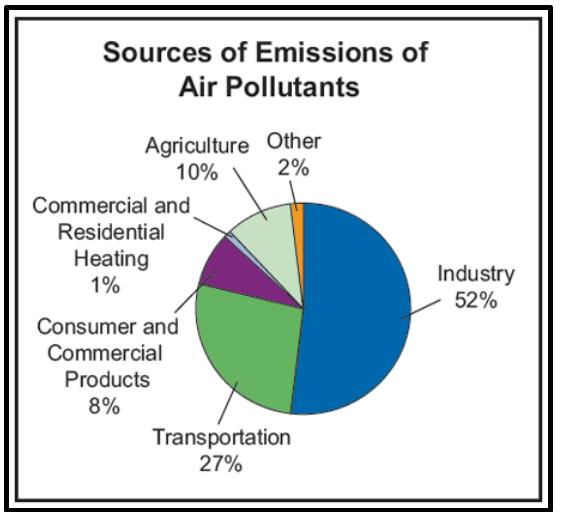
Figure 7 Causes of Air Pollution
What are the effects of Air
Pollution?
·
Acid Rains Rains often contain
acidic compounds that can affect animals, plants and crops.
·
Harmful diseases and allergies Inhalation of
harmful substances can lead to diseases such as heart problems and cancer and
allergic reactions in the skin and eyes.
·
A decrease in the visibility The suspended
particles in the air affects the visibility and also lead to the formation of
smog in the cold weather.
·
Global Warming The temperature of the
earth rises due to the presence of greenhouse gases in it such as carbon
dioxide and methane.
·
Ozone Layer Depletion - The air
pollution leads to depletion of the outer covering of the ozone layer around
the earths atmosphere.
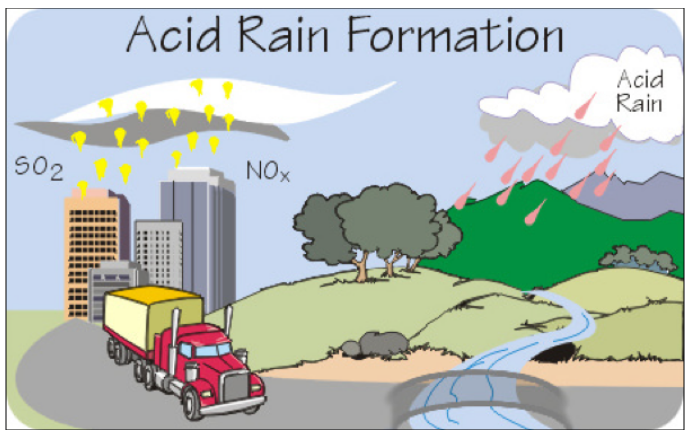
Figure 8 Acid Rain
Water
The different forms of water present on the earth
are:
·
Water Vapour found in the atmosphere
·
Saline Water found in seas and oceans
·
Freshwater found in frozen ice caps,
snow-covered mountains, underground, rivers, lakes and ponds
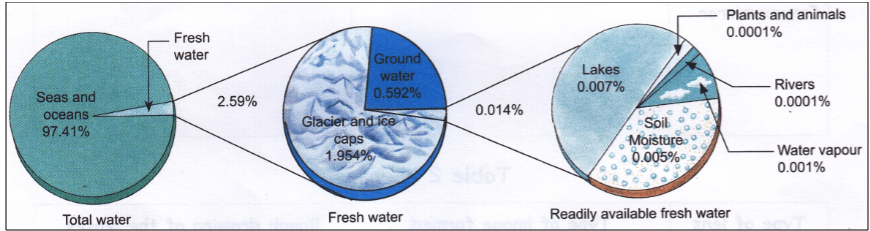
Figure 9 Water Present on Earth
Why water is a necessity for life?
·
The presence of water in a region decides the
biodiversity of that area to a great extent.
·
The cellular processes take place in the
water-like-medium.
·
A major constituent of blood is water which
allows it to carry substances throughout the body.
·
Water helps in regulating the body
temperature in animals and human beings.
·
It prevents the tissues, organs and cells
from drying out by keeping them moist.
·
Water helps in digestion of food.
·
Water helps in the removal of waste products
out of the cells.
·
Plants also require water to transport food
through different parts such as the stem and leaves and also in the process of
photosynthesis.
Water Pollution
Water pollution occurs when harmful substances such
as chemicals and waste materials like garbage are present in water that affect
its quality and the presence of life in it.
The causes of Water Pollution
·
Waste from Industries The industries often
release chemicals directly into water bodies such as rivers and seas which
contaminates it.
·
Sewage The waste produced from households
is often released into the water which gives rise to harmful bacteria in the
water.
·
Mining Activities The metal wastes obtained
from mining activities harm the organisms present in water
·
Usage of Fertilizers and Pesticides The
chemicals present in fertilizers and pesticides are extremely harmful to
aquatic animals, plants as well as animal consumption.
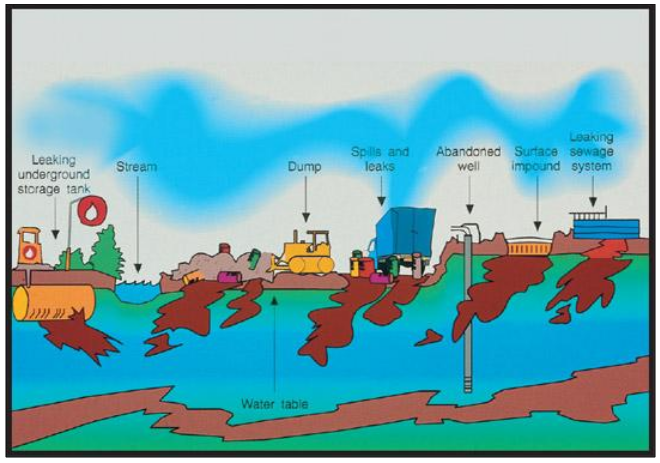
Figure 10 Causes of water pollution
The Effects of Water Pollution
·
Addition of unwanted substances such as
fertilizers pesticides and industrial wastes can make it poisonous and
extremely harmful for consumption.
·
It can also lead to an increase in bacteria
that causes severe diseases like Cholera.
·
Water pollution can lead to a decrease in the
amount of Oxygen and nutrients in the water which affects the aquatic life.
·
Water pollution can cause changes in the
temperature of water. An increase in temperature is not suitable for all the
aquatic animals especially their eggs.

Figure 11 Effects of water pollution
Soil (Land)
The upper layer of the earth called crust stores
different nutrients that can help in sustaining life on earth. But they are
generally bounded in rocks and mountains. Over a large course of time, these
rocks breakdown through some chemical, biological and physical processes which
leads to the formation of a nutrient-rich soil.
Soil Profile
·
The soil is found in several layers which are
arranged as the soil is formed.
·
The layers of the soil are also called as Horizons.
·
These layers have different types of soil
particles and colour and hands are differentiated on this basis.
·
The soil profile is defined
as the vertical section of soil that represents the sequence of layers to the
soil.
·
The layers of the soils help in understanding
the usage of that soil.
·
The soil mainly consists of four layers. Such
a soil is called Mature Soil.
·
Some types of soils consist of two layers
only. They are called Immature Soils.
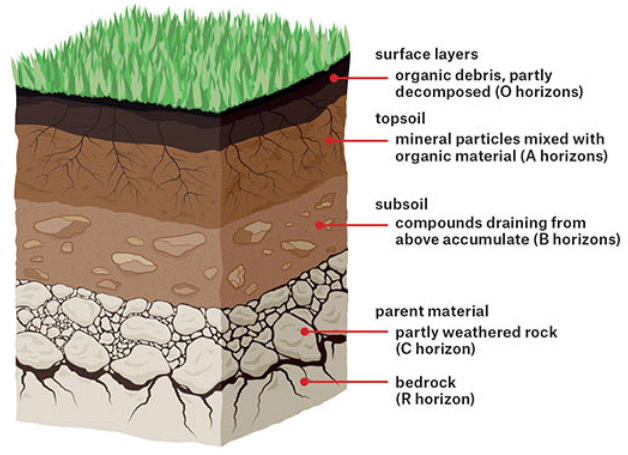
Figure 12 Soil Profile
The layers or Horizons of soil
1. The Topsoil or Horizon A or the
Humus Layer
·
This layer of soil consists of
organic matter and decomposed substances.
·
It is dark in colour, porous and can
hold air and water in good amounts.
·
Due to such quality, many living
organisms are found in the topsoil, for example, the earthworms, fungi and
bacteria.
Horizon O
Some soils contain a layer of organic matter which
consists of a large amount of decomposed leaves and humus. It is called the organic
layer of the soil.
2. Subsoil or Horizon B
·
It lies below the topsoil. It is hard and
compact than the topsoil.
·
The subsoil has a light colour because it
does not contain much humus.
·
The subsoil does not contain much organic
matter other but contains minerals in good quantity and metal salts like iron
oxide.
·
When farmers plough their field, they often
mix the topsoil and subsoil so that the crops can grow easily.
3. Horizon C or Regolith
This layer lies beneath the subsoil layer.
It is very hard and consists of stones and partly
weather pieces of rocks. There is no organic matter in this layer. The roots of
plants and trees cannot penetrate up to this layer.
4. Horizon R or Bedrock
This is the last layer of the soil which consists
of unweathered rocks
Factors Affecting
the formation of Soil
1. The sun
·
It is responsible for breaking down the rocks
into smaller pieces and forming cracks in between them.
·
The sun's radiations heat up the rocks during
the daytime. As a result, the rocks expand.
·
But during the night, these rocks cool down
and therefore contract.
·
All the parts of the rocks may not cool down
or heat up at the same time.
·
All this leads to the formation of cracks in
them and ultimately breaks them down.
2. Water
·
Water gets into the cracks of the rocks and
freezes down there.
·
This leads to whitening of the cracks.
·
Flowing water often carries pieces of rocks
away and on that path they get broken down into smaller pieces as they rub
against each other and also due to the pressure of the flowing water. This is
one of the reasons why soil is formed far away from the parent Rock.
3. Winds
·
Winds can wear down the rocks and break them.
·
Strong winds rub against the rocks and break
them or wear them down just like water.
·
Winds also carry away the soil or sand from
one place to another.
4. Living organisms
·
Lichens that can grow on the rocks and
secrete a certain substance that can powder down a rock which leads to the
formation of soil.
·
Small plants such as moss often grow on rocks
and break them down.
·
It may also happen that the roots of
different plants and trees get into the rocks surface and break it down or
widen the cracks.
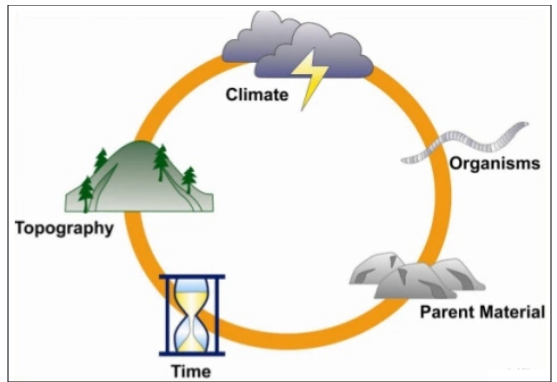
Figure 13 Factors affecting the formation of soil
Soil is a mixture of various substances It contains
the following:
·
Small pieces of rocks
·
Bits of decayed living organisms called
the Humus
·
Microscopic organisms
·
Minerals and nutrients
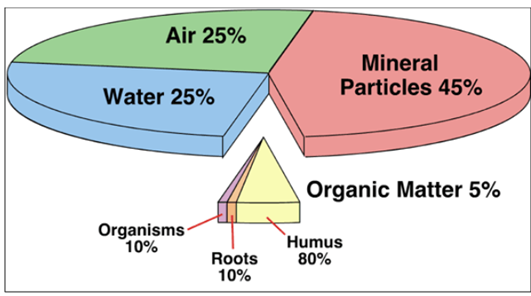
Figure 14 Composition of Soil
Factors that decide the type of Soil
·
The amount of humus present in the soil the
more the humus the more porous and deep the soil is.
·
The number of microscopic organisms in the
soil they help in keeping it fertile
·
The parent rocks they decide the minerals
that are present in the soil
All these factors also decide which kind of plants
will grow in that soil.
Topsoil The upper layer of the soil which
contains all living organisms and humus is called topsoil. The quality of
topsoil in a region decides the biodiversity of that place.
Soil Pollution
We know that soil contains different types of
substances all of them are responsible for the sustenance of the biodiversity.
When the useful components get removed from the soil, it loses its fertility
and leads to a decrease in the microscopic life in it. This phenomenon is
called soil pollution.
The causes of Soil Pollution
·
Long usage of fertilizers and pesticides
leads to the killing of the microorganisms present in it. Without these
organisms, the soil would not get recycled and replenished. Earthworms get
killed because of the pesticides. They are the ones that lead to the formation
of humus in the soil.
·
Flowing water and winds can carry away the
soil particles and often lead to exposure of rocks.
·
Deforestation can also lead to soil pollution
as the uprooting of trees exposes the soil to rains and winds.
·
Industrial activities like mining and
extraction of minerals can lead to a mixture of harmful chemicals in the soil
and decay its quality.
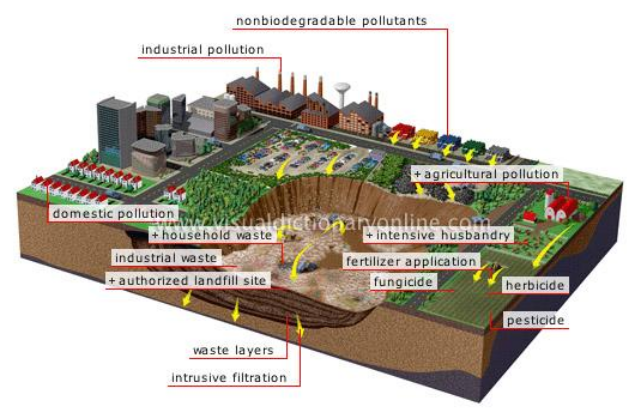
Figure 15 Soil (Land) Pollution
The effects of Soil Pollution
·
It severely affects the growth of plants.
·
It can lead to infertility of soil and thus
would restrict agriculture on such land.
·
The fertilizers decay the quality of the
soil.
·
It can affect the health of human beings who
consume food grown in soil which has large amounts of fertilizers and
pesticides mixed in it.
·
It can change the structure of soil thus
decaying the growth of useful bacteria and other microorganisms in the soil.
Soil Erosion It a process in which the upper layer
of the soil gets washed away thus leading to degradation in the soils quality.
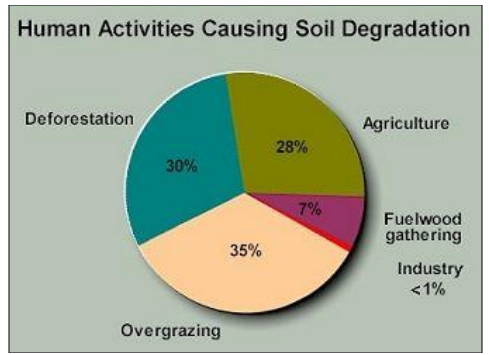
Figure 16 What Causes Soil Erosion?
How can the roots of plants prevent
soil erosion?
·
The roots of the plants bind the soil
together and prevent the winds and flowing water to sweep away the soil
particles.
·
The plants also lead to the movement of water
inside the soil and allow it to reach the deeper layer leading to an increase
in the water retention of the soil and the underground water levels.
Biochemical Cycles
A biochemical cycle refers to the natural cycle of
the earth through which a chemical substance or matter moves through the biotic
and abiotic components of the earth. These components always interact with each
other and form a stable system in the biosphere.
There are four main biogeochemical cycles
·
The Water Cycle
·
The Nitrogen Cycle
·
The Carbon Cycle
·
The Oxygen Cycle
The Water Cycle
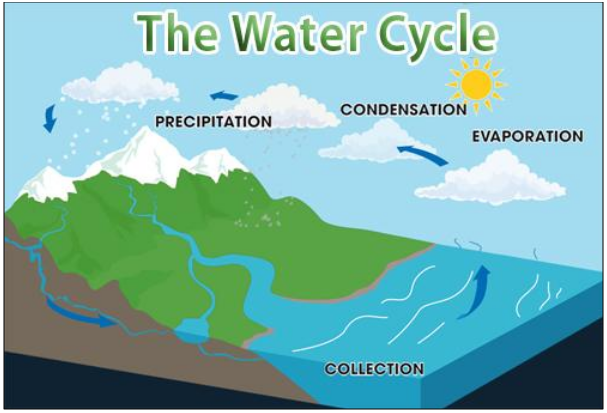
Figure 17 Water Cycle
The water cycle refers to the continuous movement
of water from the sky to the ground and then back again. The water cycle can be
divided into different stages:
·
Evaporation - Water from the Earth evaporates into
water vapours and travels up to the atmosphere.
·
Condensation The water cools
down and forms clouds up in the atmosphere.
·
Rainfall, Snowfall or Precipitation The water then
falls back to the ground
The water that falls on the earth and travels
through different pathways before it finally falls back into the sea and oceans
via rivers. Some of the water is also being utilized by the plants, animals and
human beings present on the earth. But the journey of water continues in the
form of the water cycle.
The Nitrogen Cycle
The nitrogen cycle refers to the passage of
nitrogen in the atmosphere where it transforms from simple elemental form to
complex molecules and vice versa.
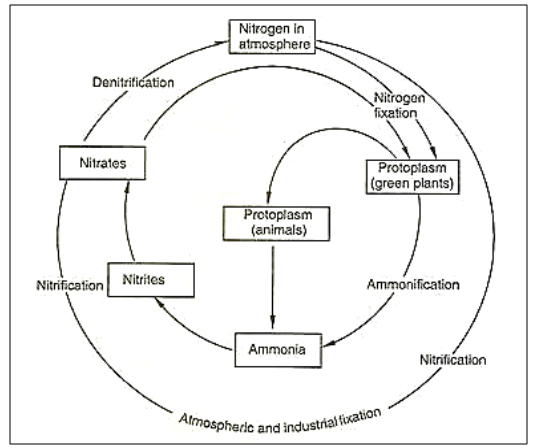
Figure 18 Nitrogen Cycle
The Importance of Nitrogen
·
It is present in abundance (78%) in the air.
·
It is an essential part of proteins and amino
acids and therefore the main component of DNA and RNA.
·
It is a common element found in alkaloids and
urea.
Different phases of the Nitrogen
Cycle
1. Nitrogen Fixation
·
Nitrogen generally exists in its inherent form
but in order to be accessed by most living organisms, it must be converted into
the organic form.
·
This process of conversion of Nitrogen into
organic form is called nitrogen fixation. There are two types of nitrogen
fixation:
i.
Biological Nitrogen Fixation
·
The biological nitrogen fixation process
takes place with the help of certain bacteria that are present in the soil, the
roots of the plants or sometimes they also live in association with the plants.
·
These microorganisms, called Nitrogen-fixing
Microorganisms, convert the nitrogen into nitrates and nitrites. For
Example, Rhizobium and Cyanobacteria called Anabaena are nitrogen fixing
bacteria
2. Physical Nitrogen
Fixation
i. Natural
Nitrogen Fixation
Another thing that allows nitrogen fixation is
lightning. The temperature and pressure on the earth rise during the lightning
which results in the formation of nitrogen oxides. These oxides dissolve in
water and form nitric acids which fall on land along with rainfall.
ii. Industrial
Nitrogen Fixation
Industrial processes such as the creation of
ammonia and nitrogen-rich fertilizers also lead to nitrogen fixation.
3. Nitrification -
The
ammonia present in the soil is then converted into nitrates and nitrites by the
bacteria. This process is called Nitrification. For Example,
Nitrobacter is a nitrifying bacterium.
4. Assimilation -
The
various nitrogen compounds are utilized by plants and animals. Plants form
amino acids with these nitrites and nitrates. The amino acids are further used
to make proteins in plants. This is the process of assimilation.
5. Ammonification -
When
the plants and animals die or excrete wastes the nitrogen present in the
organic matter enters the soil. The decomposers present in the soil break down
these nitrogen compounds into Ammonia. This process is called
ammonification. For Example, Bacillus and Clostridium are ammonifying bacteria.
5. Denitrification
In wet
soils, it becomes difficult for the microorganisms to breathe. A special type
of bacteria called the denitrifying bacteria converts the nitrate oxides
present in the soil into oxygen and nitrogen. The oxygen is utilized by the
organisms in the soil and the nitrogen is set free. This process is
called Denitrification. As a result, nitrogen is given back into
the environment. For Example, Pseudomonas and Clostridium are
denitrifying bacteria.
The Carbon Cycle
The carbon cycle refers to the process in which
carbon is exchanged among the biotic and abiotic components of the earth.
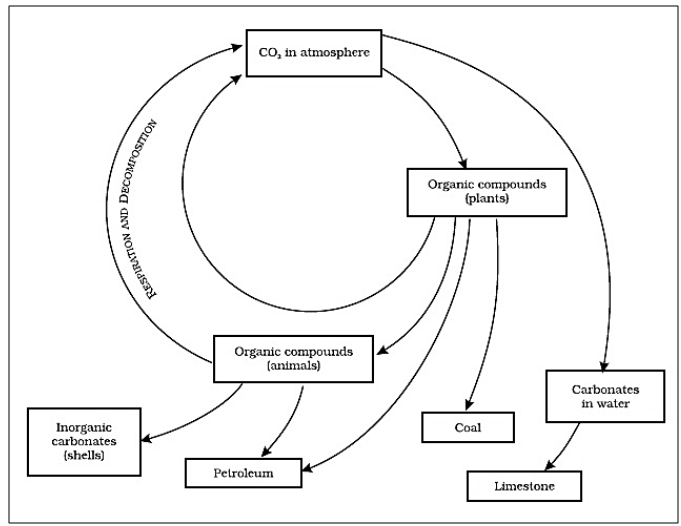
Figure 19 Carbon Cycle
Importance of Carbon
Carbon can be found on earth as
·
Carbon dioxide
·
Carbonates and hydrocarbons in minerals
·
Carbon is present in all life forms in
proteins, fats, vitamins and carbohydrates
·
The internal skeleton (endoskeleton) and
external skeleton (exoskeleton) in animals is made up of carbon salts.
·
Plants convert carbon into glucose molecules
during photosynthesis
How carbon is released back to the
atmosphere?
·
Respiration Animals utilize the glucose and gain
energy from that and then release carbon dioxide through respiration
·
Combustion Burning of fuels for different
requirements such as cooking, industrialized processes, transportation and
heating leads to the release of carbon dioxide in the atmosphere
The Greenhouse Effect
Greenhouse It is a house like
structure made up of a transparent material such as glass. This provides plants
with an appropriate environment that supports their growth, especially in the
cold regions. This is possible because the greenhouse traps the heat inside it
and prevents it from going out.
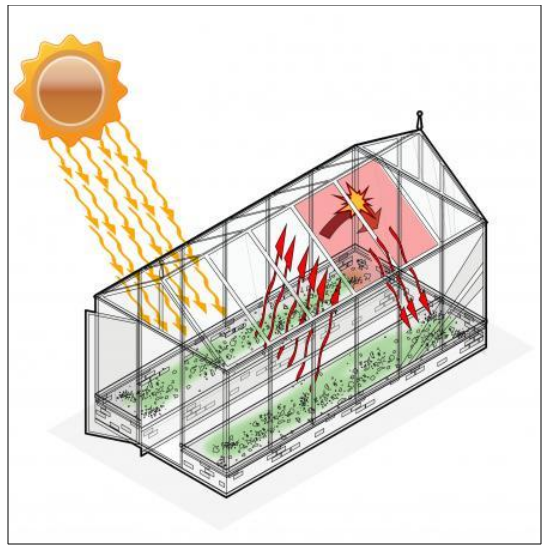
Figure 20 Greenhouse
The Greenhouse Effect - Several gases are
present in the earth's atmosphere which prevents the heat entering the
atmosphere from moving out of it. When the amount of these gases increases in
the atmosphere, the temperature of the earth rises. This leads to severe
problems like an increase in the sea levels due to the melting of the glaciers.
This whole phenomenon is termed as the greenhouse effect. (NTSE)
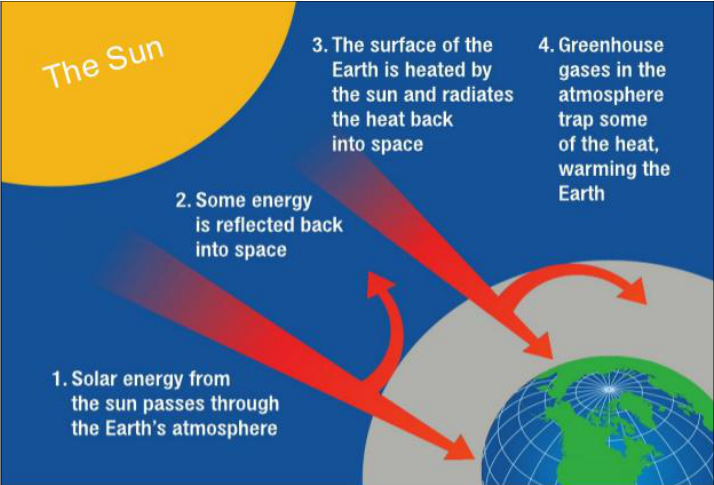
Figure 21 Greenhouse Effect on Earth
Greenhouse Gases - The gases that cause
greenhouse effect are called greenhouse gases such as carbon dioxide, methane
and ozone.
Global warming - The phenomenon in which
the average temperature of the earth increases due to the presence of
greenhouse gases is called Global Warming.
Effects of Global Warming and
Greenhouse Effect
·
Increase in the Average temperature of the
Earth - Global Warming and greenhouse effect has lead to a severe change in the climate of the earth and an
increase in the global temperature which can severely affect the biodiversity
on earth. One of the major consequences is the melting of ice caps on the earth.
·
Increase in the temperature of the
oceans - This affects the aquatic life to a great extent.
Many aquatic animals cannot survive in hot water.
·
Severe weather conditions - Many parts of the
world are facing extreme hot or extreme cold climate due to the increase in
Earth's temperature. As a result, some places receive heavy rainfall while
others face droughts.
·
Warmer Winters - The winter
season in many places on the earth has decreased due to global warming.
·
Infertile lands Many irrigational
areas can become infertile for agriculture due to change in the weather
conditions of a place. Therefore, it also affects the agricultural pattern and
availability of food all over the world.
·
Effect on animals - The migration
and hibernation of many animals are affected due to change in the climate of
the Earth. Global Warming can also lead to the extinction of many animals
because of their inability to adapt according to the changing environment.
·
Flooding of islands and coastal areas - The melting of
ice caps has resulted in increased sea levels all over the world.
·
Increase in diseases - Global warming and the
greenhouse effect lead to spread of diseases related to the heart, respiration
and eyes all over the world. Apart from these, global warming leads to infectious
diseases like cholera, malaria and dengue.
The Oxygen Cycle
Oxygen cycle refers to the maintenance of the
amount of oxygen present in the atmosphere.
Importance of oxygen
·
It is the second most abundant gas present in
the atmosphere (21%).
·
It is found in the earth's crust as oxides in
metals and minerals.
·
It is found in combined form in carbon
dioxide
·
It is also found in biological compounds like
proteins, nucleic acids, carbohydrates and fats.
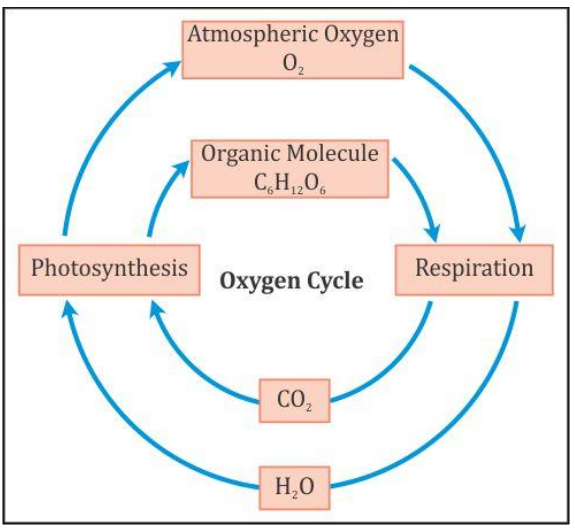
Figure 22 Oxygen Cycle
Oxygen is required in three processes:
·
Combustion
·
Respiration
·
Formation of Nitrogen oxides
Oxygen is returned back to the environment by the
process of photosynthesis only. That is why it is very
important to grow more and more plants so that more amount of oxygen can be
returned back to the atmosphere.
The Ozone Layer
The upper layer of the atmosphere of the Earth is
covered by a gas called Ozone (O3). This gas is poisonous in nature
but it doesn't affect the life as it is found far away from the biosphere.
The Ozone Layer acts as a shield and prevents the
harmful ultraviolet rays of the sun from reaching the Earth's surface. In this
way, it allows the survival of life on earth.
The Ozone layer depletion
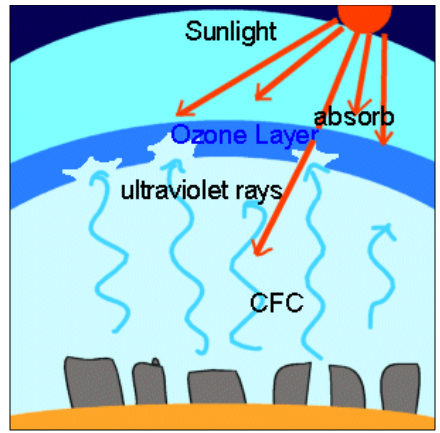
Figure 23 Ozone Layer Depletion
It has been discovered that the Ozone Layer is
getting depleted from the earth's atmosphere. A hole in Ozone Layer has been
found over Antarctica.
·
This is mainly because of the presence of
the Chlorofluorocarbons (CFCs) in the atmosphere. These are
released due to industrial processes, refrigeration, aerosols, foams and
solvents.
·
The chlorine and fluorine present in these on
reaching the Ozone Layer react with it to form complex compounds and it thus
results in depletion of Ozone.
·
If the Ozone Layer would deplete the
ultraviolet rays of the sun would under the Earths atmosphere which could lead
to severe effects such as:
o
An increase in the risk of having skin cancer
o
Damaging the eyes
o
The weakening of the immune systems
o
Skin allergies
o
Decay in the growth of plants and animals
o
Breakdown of the natural carbon cycle