Structure of Atom
·
Atoms are the basic building blocks of
matter.
·
Different kinds of matter exist because
there are different kinds of atoms present in them.
Charged Particles in Matter
·
Whenever we rub two objects together, they
become electrically charged. This is because atoms contain charged particles in
them. Therefore, atoms can be divided further into particles i.e proton, electron and neutron.
Protons were discovered by Ernest Rutherford, in
his famous gold foil experiment.
Electrons were discovered by J.J. Thomson, in his
cathode ray tube experiment.
Neutrons were discovered by James Chadwick.
·
Atoms consist of protons and electrons
in a balanced proportion.
·
Protons exist in the interiors of the atom
and electrons exist in the exteriors of the atom. Therefore, electrons can be
removed from an atom.
Failure of Dalton’s Atomic Theory
The postulates of the atomic theory by John Dalton
·
The matter is made up of tiny particles
called Atoms that cannot be divided.
·
Atoms are never formed or destroyed
during a chemical reaction.
·
Atoms of an element exhibit same
nature. They have the same size, mass, and character.
·
Atoms of different elements exhibit
variant nature. They do not have same characteristics.
·
Atoms form compounds by combining in a
ratio of whole numbers.
·
A compound contains a constant number
and kinds of atoms
Dalton suggested that atoms can neither be created
nor destroyed and are indivisible. But the discovery of electrons and protons
in atoms lead to failure of this aspect of Dalton’s theory.
Thomson’s Model of an Atom
According to J.J. Thomson, the structure of an atom
can be compared to Christmas pudding where electrons are present inside a
positive sphere.
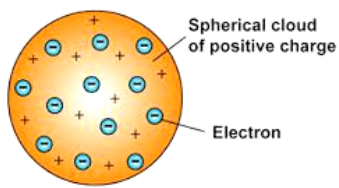
An atom is composed of a positively charged sphere
in which electrons are embedded.
Atom is neutral as the positive and negative
charged are equal in proportion.
Rutherford’s Model of an Atom
Rutherford’s Experiment
·
He experimented with thin gold foil by
passing alpha rays through it.
·
He expected that the gold atoms will
deflect the Alpha particles.
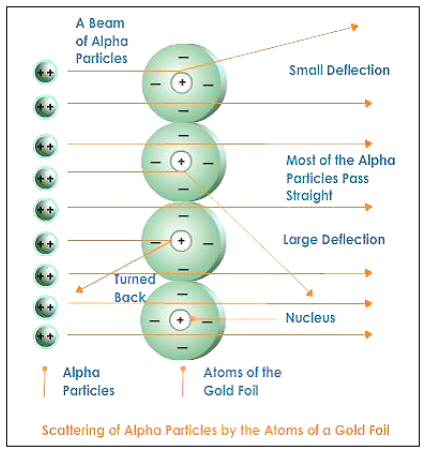
|
Observations |
Inferences |
|
Alpha particles which had high speed
moved straight through the gold foil |
Atom contains a lot of empty space |
|
Some particles got diverted a by
slide angles |
Positive charges in the atom are not
occupying much of its space |
|
Only one out of 12000 particles
bounced back |
The positive charges are concentrated
over a particular area of the atom. |
Thus, Rutherford gave the nuclear model of an atom
based on his experiment which suggests that -
·
Atoms contain a lot of unoccupied space
·
There is a heavily positively charged
substance present in the center of the atom which is
called the nucleus
·
The nucleus contains an equal amount of
positive and negative charge.
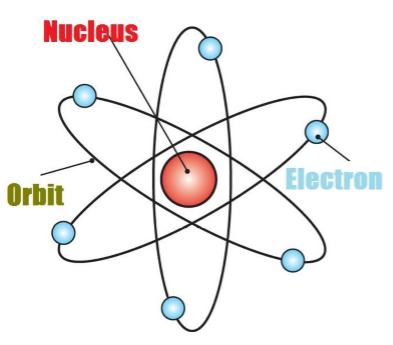
The Nucleus of an Atom
·
The nucleus id located at the center of the atom.
·
All the mass of the atom is because of
the nucleus.
·
The electrons revolve around the
nucleus in circular parts which are called Orbits
·
If we compare the size of the atom and
nucleus, the nucleus is much smaller than the atom.
Drawbacks of the Nuclear Model of an
Atom
The Nuclear Model of the Atom failed to explain how
an atom remains stable despite having positive and negative charges present in
it. Maxwell has suggested a theory according to which if any charged particle
moves in a circular motion it radiates energy. So, if electrons start moving in
a circular motion around the nucleus they would also radiate some energy which
would decrease at the speed of the electrons. As a result, they would fall into
the nucleus because of its high positive charge.
What are nucleons?
Protons and Neutrons are collectively called
as Nucleons.
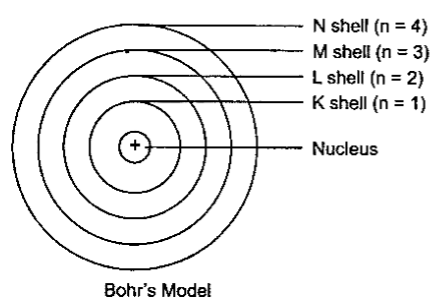
Bohr's Model of an Atom
Bohr suggested that –
·
Electrons spin around the nucleus in an
individualized separate path or unattached orbit.
·
The electrons do not emit any energy
while moving Indies special orbits.
·
These orbits are also called as Energy
Levels.
·
They are represented using letters or
numbers as shown in the figure below –
The Neutrons
J. Chadwick discovered that there is another
sub-atomic particle present in the atom. This particle carries no charge and is
known as a Neutron. Therefore, we can conclude that atom consists
of three types of particles -
|
Electrons |
which carry a negative charge |
|
Protons |
which carry a positive charge |
|
Neutrons |
they are neutral |
The distribution of electrons in different shells
or orbits
·
If Orbit number = n
·
Then number of electrons present in an
Orbit = 2n2
·
So, for n =1
·
Maximum electrons present in shell – K
= 2 * (1)2 = 2
·
The outermost shell can contain at most
8 electrons.
·
The shells in an atom are filled in
sequence.
·
Thus, until the inner shells of an atom
are filled completely the outer shells cannot contain any electrons.
Valency
·
Valence Electrons – Electrons
existing in the outermost orbit of an atom are called Valence Electrons.
·
The atoms which have completely filled
the outermost shell are not very active chemically.
·
The valency
of an atom or the combining capacity of an atom is given by the number of
elements present in the outermost shell.
·
For Example, Helium contains
two electrons in its outermost shell which means its valency
is two. In other words, it can share two electrons to form a chemical bond with
another element.
·
What happens when the outermost shell
contains a number of electrons that are close to its maximum capacity?
Valency in such cases is generated
by subtracting the number of electrons present in the outermost orbit from
octet (8). For example, oxygen contains 6 electrons in its outermost shell. Its
valency is calculated as: 8 – 6 = 2. This means
oxygen needs two electrons to form a bond with another element.
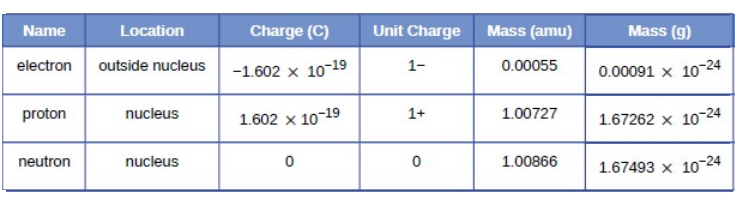
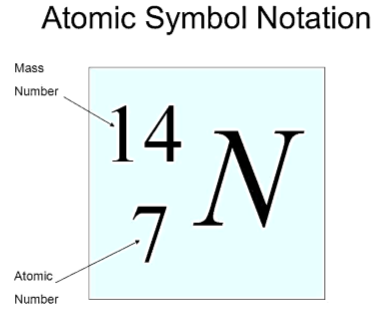
Atomic Number of an Element
Atomic Number (Z) = Number of protons in an
atom
Mass Number of an Element
Mass Number = Number of protons + Number of
neutrons
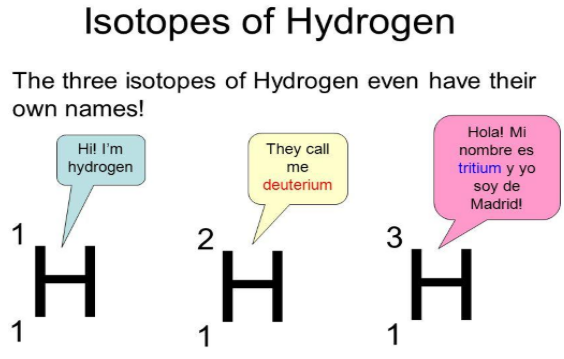
Isotopes
·
The atoms of an element can exist in
several forms having similar atomic numbers but varying mass numbers.
·
Isotopes are pure substances.
·
Isotopes have a similar chemical
nature.
·
Isotopes have distinct physical
characteristics.
Where can we use Isotopes?
1. The fuel of Nuclear Reactor – Isotope of Uranium
2. Treatment of Cancer – Isotope of Cobalt
3. Treatment of Goiter –
Isotope of Iodine
Example: Consider two atomic species
namely U and V. Are they isotopes?
|
|
U |
V |
|
Protons |
5 |
5 |
|
Neutrons |
5 |
6 |
|
Mass Number |
5 + 5 = 10 |
5 + 6 = 11 |
|
Atomic Number |
5 |
5 |
From the above example, we can infer that U and V
are isotopes because their atomic number is the same.
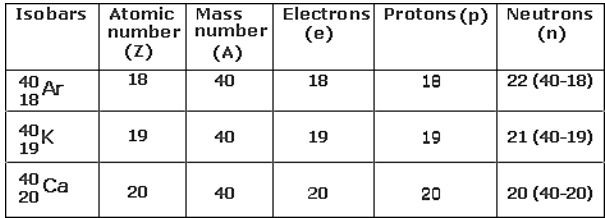
Isobars
The atoms of several elements can have a similar
mass number but distinct atomic masses. Such elements are called Isobars.