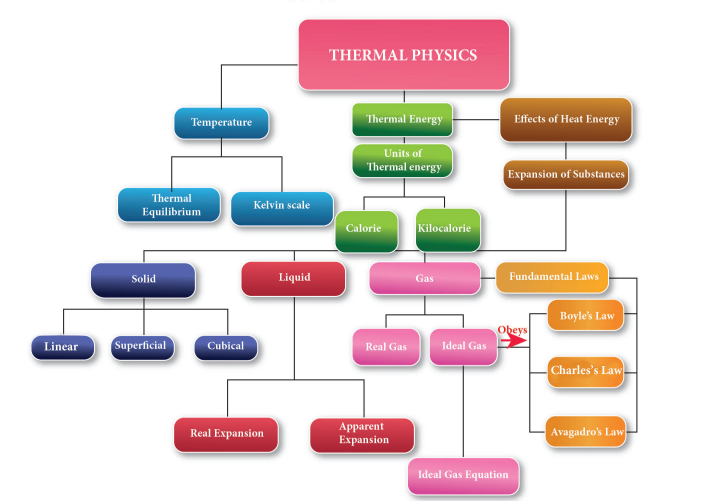
THERMAL PHYSICS
Ø Thermal physics is
the combined study of thermodynamics, statistical mechanics, and kinetic theory
of gases.
Ø This
umbrella-subject is typically designed for physics students and functions to
provide a general introduction to each of three core heat-related subjects.
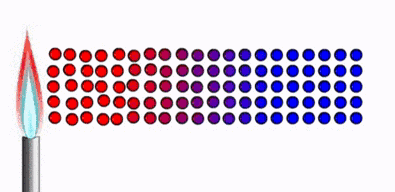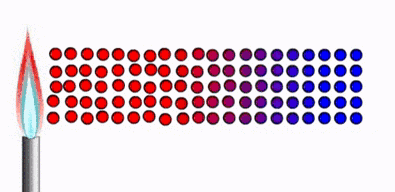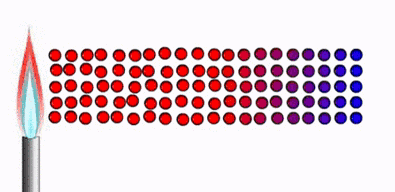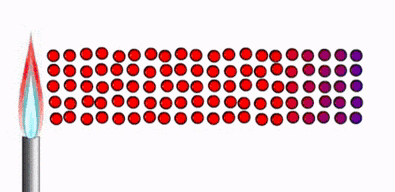
Thermal
equilibrium :
Ø Two physical systems
are in thermal equilibrium if there is no
net flow of thermal energy between them when they are connected by a path
permeable to heat.

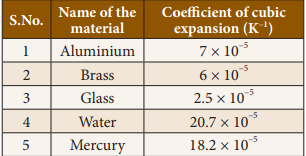
Coefficient
of cubical expansion of some materials :
FUNDAMENTAL
LAWS OF GASES :
Ø The three
fundamental laws which connect the relation between pressure, volume and
temperature are as follows:
1) Boyle’s Law
2) Charles's law
3) Avogadro's law
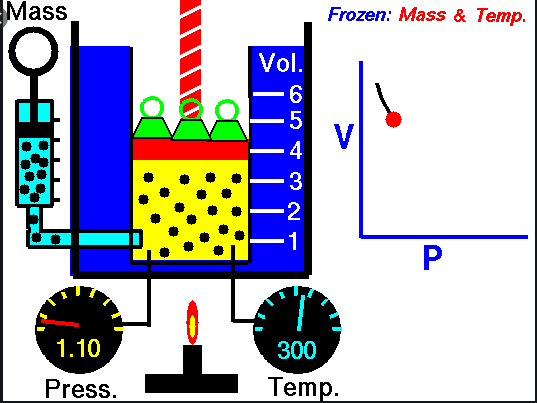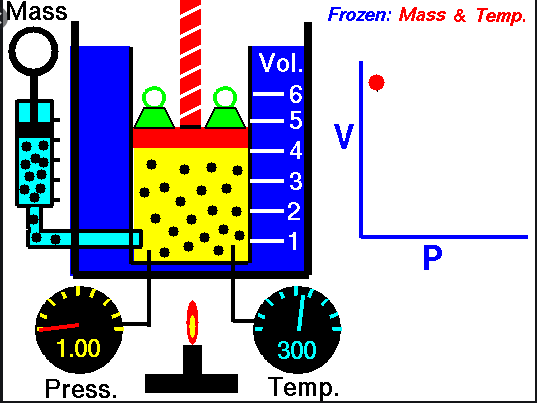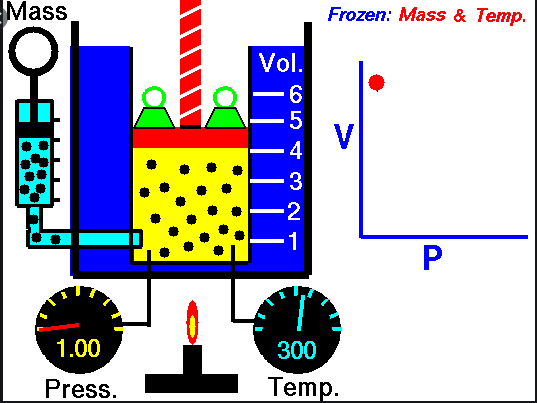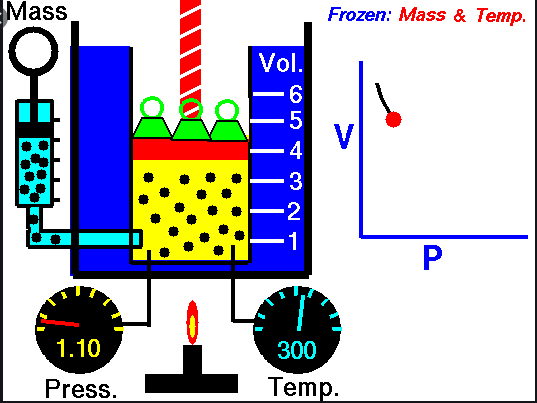
Boyle’s
Law –
Ø When the
temperature of a gas is kept constant, the volume of a fixed mass of gas is
inversely proportional to its pressure.
Charles's
law -
Ø Charles’s
law was formulated by a French scientist Jacques Charles.
Ø According
to this law, When the pressure of gas is kept
constant, the volume of a gas is directly proportional to the temperature of
the gas.

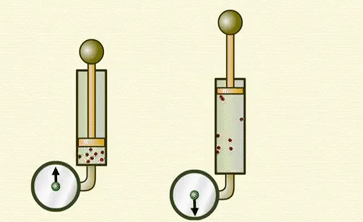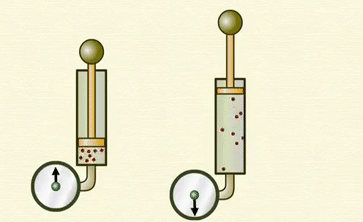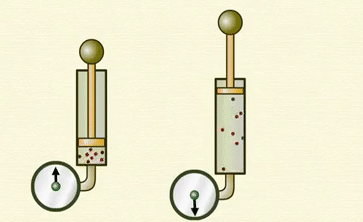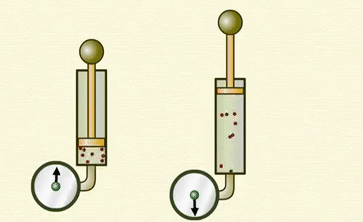
Avogadro's
law -
Ø Avogadro's
law states that at constant pressure and temperature, the volume of a gas is
directly proportional to number of atoms or molecules present in it.
MIND MAP