ATOMS AND MOLECULES
Atoms:
Ø It is the smallest
constituent unit of matter that possess the properties of the chemical element.
Ø Atoms don’t exist
independently, instead, they form ions and molecules which further combine in
large numbers to form matter that we see, feel and touch.
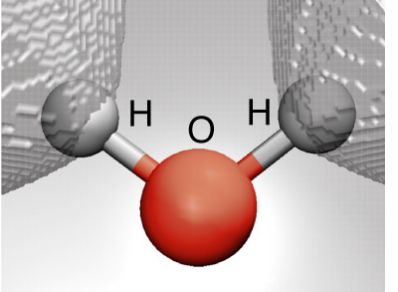
Molecules:
Ø A molecule is
defined as the smallest unit of a compound that contains the chemical
properties of the compound.
Ø Molecules are made
up of groups of atoms.
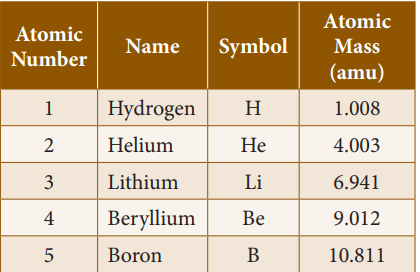
Relative atomic mass of elements (C-12
Scale):

Atomic mass of some elements:
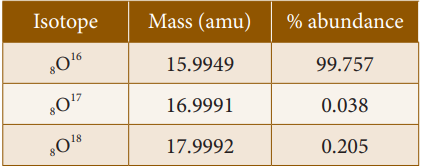
Isotopes of oxygen:
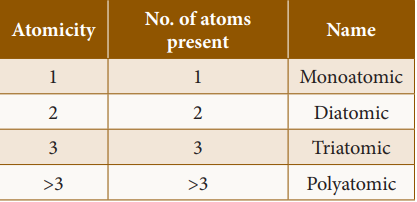
Classification of molecules:
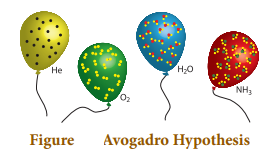
AVOGADRO HYPOTHESIS:
Ø The Avogadro’s law states that “equal volumes
of all gases under similar conditions of temperature and pressure contain equal
number of molecules”.