PERIODIC CLASSIFICATION OF ELEMENTS
MODERN
PERIODIC TABLE:
Ø The modern periodic table is a tabular
arrangement of elements in rows and columns, highlighting the regular
repetition of properties of the elements.
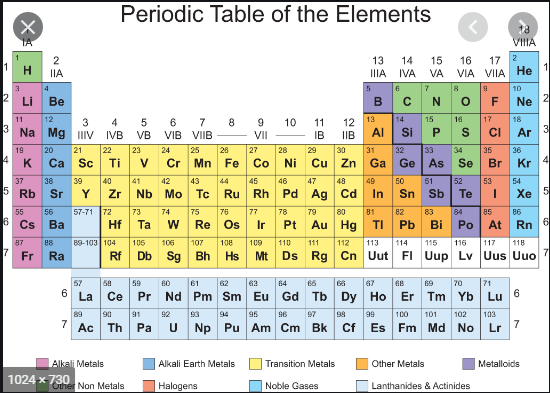
Features
of Periods:
Ø The horizontal rows are called periods. There
are seven periods in the periodic table. ‹
First period (Atomic number 1 and 2): This is the shortest period. It contains
only two elements (Hydrogen and Helium).
Ø Second period (Atomic number 3 to 10): This
is a short period. It contains eight elements (Lithium to Neon).
Ø Third period (Atomic number 11 to 18): This
is also a short period. It contains eight elements (Sodium to Argon).
Ø Fourth period (Atomic number 19 to 36): This
is a long period. It contains eighteen elements (Potassium to Krypton). This
includes 8 normal elements and 10 transition elements.
Ø Fifth period (Atomic number 37 to 54): This
is also a long period. It contains 18 elements (Rubidium to Xenon). This
includes 8 normal elements and 10 transition elements.
Ø Sixth period (Atomic number 55 to 86): This
is the longest period. It contains 32 elements (Caesium to Radon). This
includes 8 normal elements, 10 transition elements and 14 inner transition
elements (Lanthanides).
Ø Seventh period (Atomic number 87 to 118):
Like the sixth period, this period also accommodates 32 elements. Recently 4
elements have been included by IUPAC.
Ø
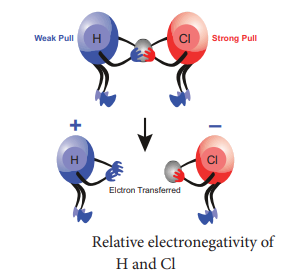
Electronegativity:
Ø Electronegativity of an element is the
measure of the tendency of its atom to attract the shared pair of electrons
towards itself in a covalent bond.
Types
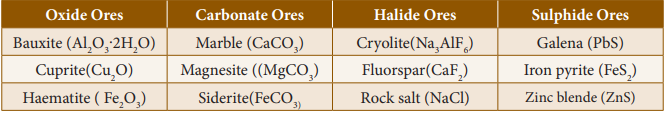
of ores:
ALLOYS:
Ø An alloy is a homogeneous mixture of two or more metals
or of one or more metals with certain non-metallic elements.
Types of
Alloys -
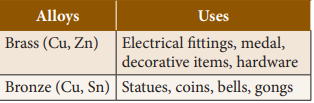
1.
Copper Alloys
(Non- ferrous)
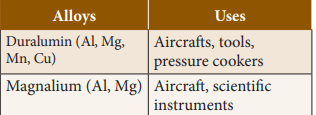
2.
Aluminium Alloys
(Non- ferrous)
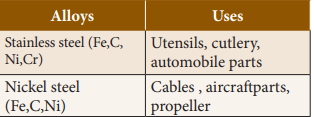
3.
Iron
Alloys(Ferrous)
MIND MAP