SOLUTIONS
Ř A homogenous mixture of two
or more substances in relative amounts that can be varied continuously up to
what is called the limit of solubility.
Ř The term solution is
commonly applied to the liquid state of matter, but solutions
of gases and solids are possible.
Ř Air, for example, is a solution consisting chiefly of oxygen and nitrogen with trace amounts of several
other gases, and brass is a solution composed of copper and zinc.
Homogeneous
mixtures:
Ř A homogeneous
mixture is a solid, liquid, or gaseous mixture that
has the same proportions of its components throughout any given sample.

Heterogeneous
mixtures:
Ř Conversely,
a heterogeneous mixture has components in
which proportions vary throughout the sample.

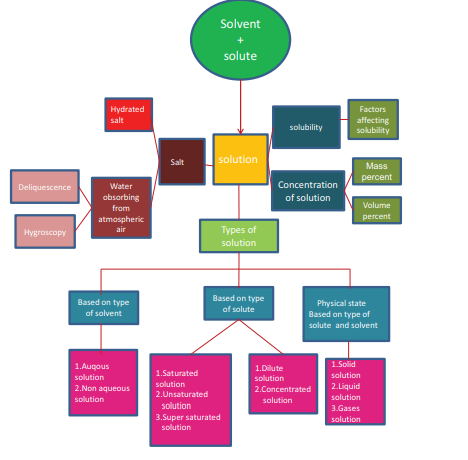
Types
of binary solutions:
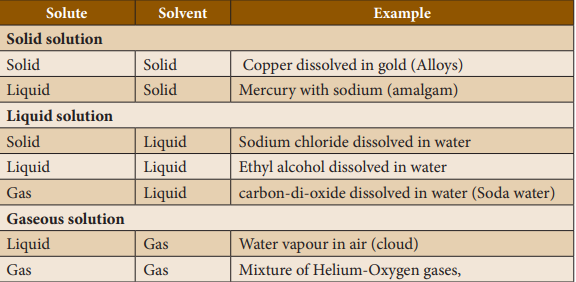
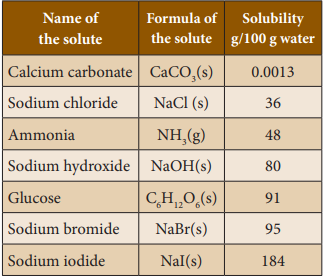
Solubility’s
of some common substances in water at 25°C –
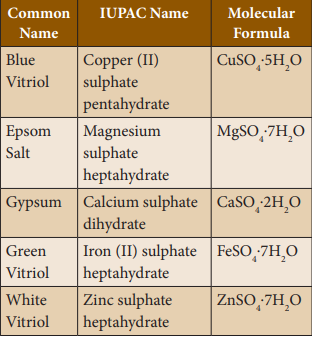
Hydrated
salts:
Difference
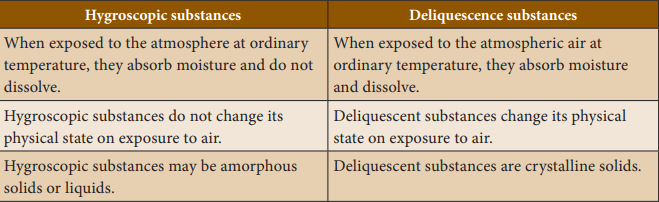
between hygroscopic substances and deliquescence:
MIND MAP