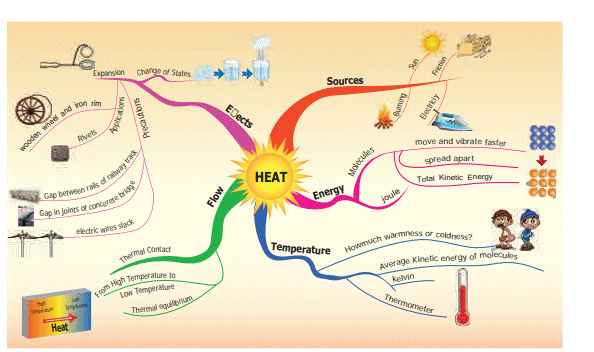
Heat
Introduction
We
are all familiar with heat. We feel it on our body when the sun shines, we use
heat for cooking our food, We reduce the heat by adding ice cubes
while preparing fruit juice. Let us learn about sources of heat.
1.1 Sources of heat
❏
Sun

We
all know that the sun gives us light. Does it give us heat? After standing
under the sun light for some time, touch your head. Does it feel hot? Yes, it
feels hot because the sun gives out heat besides light. Now, you can understand
why it is difficult to walk bare-footed on sunny days in the afternoon.
❏
Combustion (Burning)

Heat
energy can be generated by the burning of fuels like wood, kerosene, coal,
charcoal, gasoline/petrol, oil, etc., In your home,
how do you get heat energy to cook food?
❏
Friction
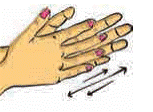
Rub
your palms for some time and then hold them to your cheeks. How do you feel? We
can generate heat by rubbing two surfaces of some substances. In the past
people used to rub two stones together to light fire.
❏
Electricity

When electric current flows through a
conductor, heat energy is produced. The water heater, iron box, electric kettle
etc., work on this principle.
1.2 Heat
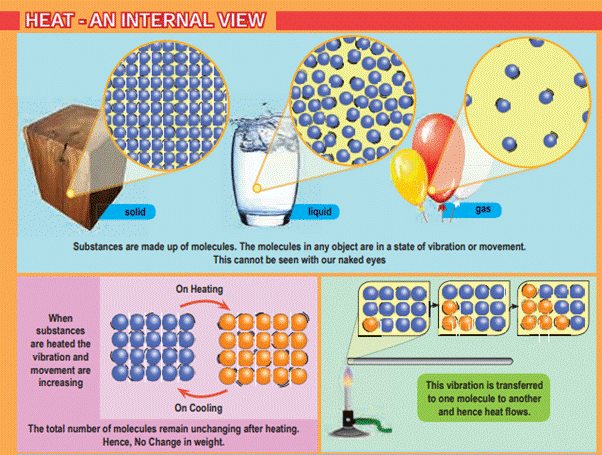
Molecules
in objects are constantly vibrating or moving inside objects. We cannot see
that movement with our naked eye. When we heat the object this vibration and
movement of molecules increases and temperature of the object also increases.
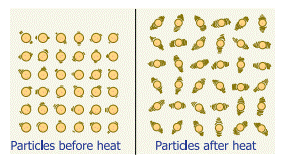
Ø Thus,
Heat is an energy that raises the temperature of a thing by causing the
molecules in that thing to move faster.
Ø Heat
is not a matter. It doesn’t occupy space. It has no weight. Like light, sound
and electricity, heat is a form of energy.
Ø In
short, Heat is the total kinetic energy of constituent particles of objects. SI
Unit of Heat is joule. The unit calorie is also used.
1.3 Hot and cold objects
In
our day-to-day life, we come across a number of objects. Some of them are hot
and some of them are cold. How do we decide which object is hotter than the
other?
We use the tip of our finger to
find out whether the tea in a cup has enough heat to drink or whether milk has
been cooled enough to set for making curds. We often determine heat by touching
the objects. But is our sense of touch reliable?
1.4 Temperature
Definition
of Temperature: The measurement of
warmness or coldness of a substance is known as its Temperature.
SI
unit of temperature is kelvin. Celsius and Fahrenheit are the other units used.
Celsius is called as Centigrade as well.
It
determines the direction of flow of heat when two bodies are placed in contact.
1.5 Heat
and Temperature
Heat
and temperature are not the same thing, they in fact mean two different
things;
·
Temperature is related to how fast the
atoms or molecules move or vibrate within the substance.
·
Heat not only depends on the
temperature of the substance but also depends on how many molecules are there
in the object.
·
Temperature measures the average
kinetic energy of molecules. Heat measures the total Kinetic Energy of the
molecules in the substance.
Total
heat is measured by calorie, the amount of heat needed to raise one gram of
water by one degree centigrade.
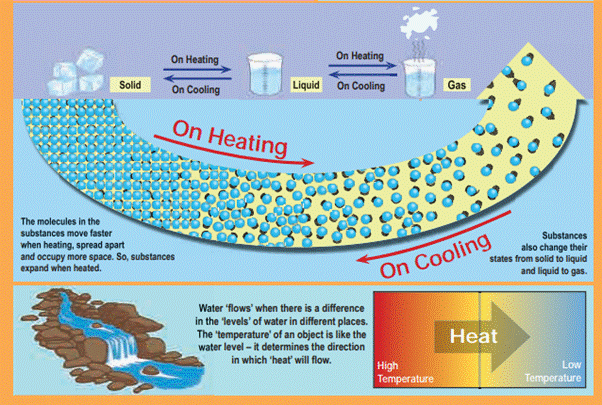
1.6 Flow of Heat
An analogy between temperature and water
level:
Water
‘flows’ when there is a difference in the ‘levels’ of water in different
places. It does not matter if there is more water in one place or another.
Water from a puddle can flow into a reservoir or the other way around. The
‘temperature’ of an object is like the water level – it determines the
direction in which ‘heat’ will flow. Heat energy flows from higher temperature
to lower temperature.
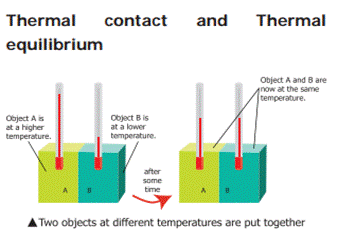
Consider
two bodies A and B. Let the temperature of A be higher than that of B. On
bringing bodies A and B in contact, heat will flow from hot body A to the cold
body B. Heat will continue to flow till both the bodies attain the same
temperature.
The temperature determines the
direction of flow of heat.

1.
You are holding a hot cup of coffee.
Would the Heat energy transfer from
a.
Your body to the coffee, or
b.
The coffee to your body?
2.
You are standing outside on a summer
day. It is 40o C outside (note that normal body temperature is 37o C). Would
the Heat energy transfer from.
a. Your body to the air particles, or
b. The air particles to your body?
3. You are standing outside on a winter day. It is 23o C
outside. Would the heat energy transfer from:
a.
Your body to the air particles, or
b.
The air particles to your body?
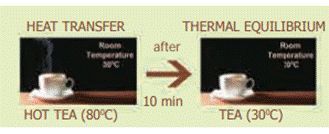
Two objects are said to be in thermal
contact if they can exchange heat energy. Thermal equilibrium exists when two
objects in thermal contact no longer affect each other's temperature.
For
example:
If a pot of milk from the refrigerator is set
on the kitchen table, the two objects are in thermal contact. After certain
period, their temperatures are the same, and they are said to be in thermal
equilibrium.
1.7 Expansion in solids
Sam is trying to open a tight jar, but
he cannot open it. He asks his uncle to help. His uncle says that pour some hot
water on the lid of the jar. Sam does so and tries to open it now. Wow! The jar
is opened easily!
Do you have such experience? How do you open a
tightly closed cap of the pen which could not be opened by you normally?
·
Most substances expand when heated and
contract when cooled. The change in length / area or volume (due to contraction
/ expansion) is directly related to temperature change.
·
The expansion of a substance on heating
is called, the thermal expansion of that substance.
1.8
Linear and Cubical Expansion
A solid has a definite shape, so when a solid
is heated, it expands in all directions i.e., in length, area and volume, all
increase on heating.
The
expansion in length is called linear expansion and the expansion in volume is
called cubical expansion.
Why
is the iron rim of a bullock cart wheel heated before it is fitted onto the
wheel? Why is a small gap left between two lengths of railway lines?
We
can perform an interesting experiment to find out an answer to these questions.
All we need to do is to heat a cycle spoke.
1.9 Uses of Thermal
Expansion
❏
Fitting the iron rim on the wooden wheel
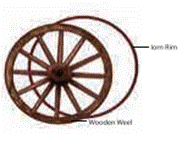
The diameter of the iron ring is slightly less
than that of the wooden wheel. Therefore, it cannot be easily slipped on from
the rim of wooden wheel.
The
iron ring is, therefore, first heated to a higher temperature so that it
expands in size and the hot ring is then easily slipped over to the rim of the
wooden wheel. Cold water is now poured on the iron ring so that it contracts in
size and holds the wooden wheel tightly.
❏
Riveting

Rivets are used to join two steel plates
together. Hot rivet is driven through the hole in the plates. One end of the
rivet is hammered to form a new rivet head.
When
cooled, the rivet will contract and hold the two plates tightly together.
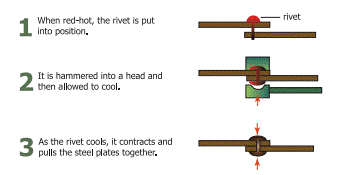
1.10 Thermal Expansion Examples
❏
Cracking of a thick glass tumbler
Glass
is a poor conductor of heat. When hot liquid is poured into the tumbler, the
inner surface of the tumbler becomes hot and expands while the outer surface
remains at the room temperature and does not expand. Due to this unequal
expansion, the tumbler cracks.
❏
Electric wires
Electric wires between electric posts
contract on cold days and sag in summers. To solve this problem, we leave wires
slack so that they are free to change length.
1.11 Numerical problems
1. I
put a kettle containing 1 liter of cold water on the gas stove, and it takes 5
minutes to reach the boiling point. My friend puts on a small electric kettle,
containing ½ liter of cold water, and it takes 5 minutes to get up to boiling
point. Which gives more heat in 5 minutes?
a.
The gas supply; or
b.
The electricity supply? Can you say how many times as much?
2.
One calorie heat energy is needed to raise the temperature of the water from
30o C to 31o C. How much heat energy is needed to raise the temperature of the
water from 30o C to 35o C.
Points to remember
1.
The main source of heat is sun, we can
obtain heat from combustion, friction, and electricity.
2.
Heat is an energy that raises the
temperature of a thing by causing the molecules in that thing to move
faster
3.
Heat is the total Kinetic energy of
constituent particles of objects.
4.
SI unit of Heat is joule (J).
5.
The measurement of warmness or coldness
of a substance is known as its temperature.
6.
SI unit of temperature is kelvin.
7.
Temperature determines the direction of
flow of heat when two bodies are placed in contact.
8.
Two objects are said to be in thermal
contact if they can affect each other’s temperature.
9.
Thermal equilibrium exists when two
objects in thermal contact no longer affect each other’s temperature.
10. Most
substances expand when heated and contract when cooled. The expansion of a
substance on heating is called the thermal expansion of that substance.
11. A
solid has a definite shape, so when a solid is heated, it expands in all
directions i.e., in length, area and volume, all increase on heating.