Matter
1.
Matter can be defined as anything, which
occupies space or volume and mass and can be perceived by our senses.
2.
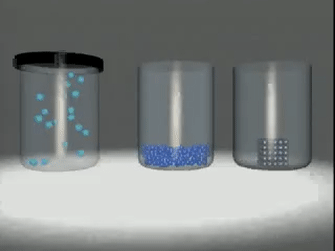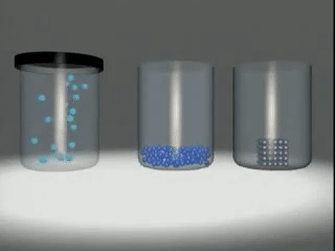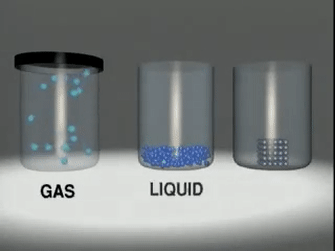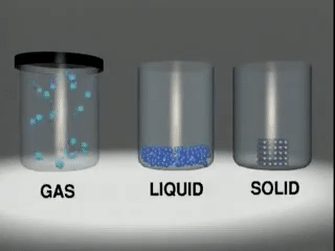
Matter exists in three
states:
◦ Solids:
Substances like wood, stone, sand,
iron etc.
◦ Liquids:
Substances like water, milk, fruit
juice, etc.
◦ Gases:
Substances like oxygen, nitrogen,
carbon dioxide, steam, etc.
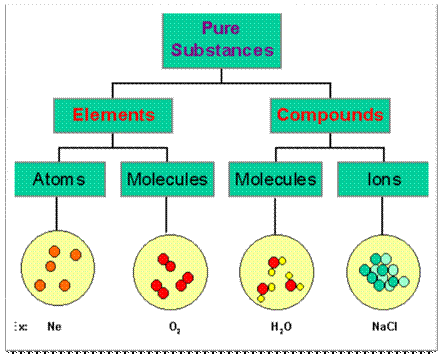
3.
Matter in any physical state is composed
of smaller particles such as atom, molecules or ions.
◦ Atom:
An atom is
the smallest particle of an element, which exhibits all the properties of that
element. It may or may not exist independently but takes part in every chemical
reaction.
◦ Molecules:
Atoms of the
same element or different elements combine to form a molecule. A molecule is
the smallest particle of a pure substance (element or compound), which can
exist independently and retain the physical and chemical properties of the
substance.
◦ Ions:
Atoms or
group of atoms having a charge (positive or negative) are called ions.
4.
A symbol is an image, object, etc., that
stands for some meaning.
5.
History of symbols of elements
◦ Greek symbols:
The
symbols in form of the geometrical shapes were those used by the ancient Greeks
to represent the four basic elements around us such as earth, air, fire and
water.
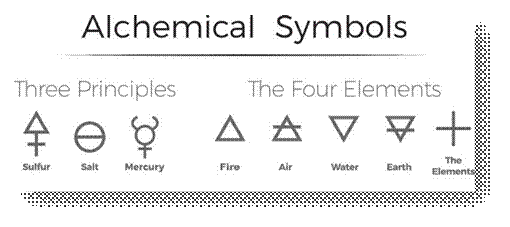
◦ Alchemist symbol:
In the days of alchemists, the different materials that they
used were represented by the above-mentioned symbols while they try to change less
valuable metal into gold. The process was called alchemy and the men who did
this work were known as alchemists.
◦ Dalton symbols:
In 1808, John Dalton, English scientist tried to name the various
elements based on these pictorial symbols. These symbols are difficult to draw
and hence they are not used today.
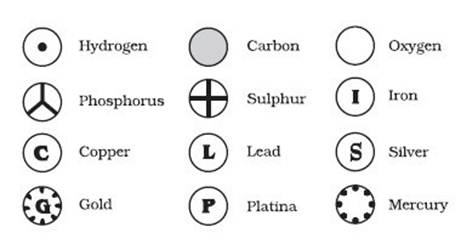
◦ Berzelius symbols:
In 1813, Jon Jakob
Berzelius devised a system using letters of alphabet rather than signs.
6.
Present System for Determining Symbols of
the Elements
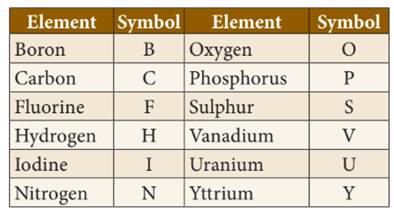
◦ The
symbols of the most common elements, mainly non-metals, use the first letter of
their English name. It is written as a capital letter.
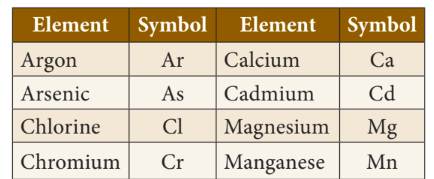
◦ If the name of the element has the same initial letter as another
element, then symbol uses the first and second letters of their Element name.
First letter in upper case and the second letter is in lower case.
◦ If the first two letters of the names of elements are the
same, then the symbol consists of first letter and second or third letter of
English name that they do not have in common.
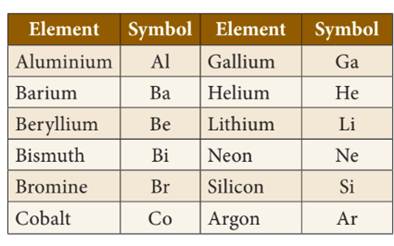
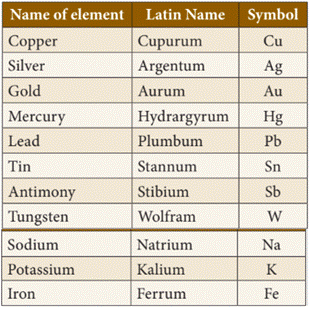
◦ Some
symbols are used on the basis of their old names or Latin name of an element.
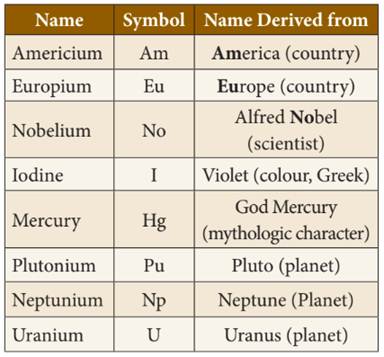
◦ Some
elements are named using name of country/scientist/color/mythological character/planet.
7.
A symbol of an element signifies
◦ Name of the element
◦ One atom of the element For example, The symbol O stands for
the element of Oxygen, or rather one atom of oxygen
8.
The wealth of a country is measured by
the amount of gold in its reserve.
9.
Elements are classified into metals,
non-metals, and metalloids based on their properties
10.
Physical Properties of Metal:

◦ Physical state:
Metals
are solid under normal conditions of temperature and pressure.
◦
Exception: Mercury is liquid at
room temperature. Elements cesium (Cs), rubidium (Rb), Francium (Fr) and
Gallium (Ga) become liquid at or just above room temperature.
◦
Hardness: Most metals are hard.
◦
Exception: Sodium and potassium are
soft enough to be cut by a knife. Osmium is so hard that it can scratch glass.
◦
Luster: All metals are shiny.
The typical shine of metals is called metallic lustre.
◦
Exception
is calcium.
◦
Density: Metals generally have
high density.
◦
Exception: Sodium and potassium
have exceptionally low density.
◦
Melting point and boiling point: Metals
in general have high melting point and boiling point. Exception: Sodium,
potassium, mercury and gallium are exceptions.
◦
Tensile strength: Metals have the capacity
to withstand strain without breaking. This property is called tensile strength.
◦
Use: It is the property that
owes the use of iron for the construction of railway tracks.
◦
Exceptions: Zinc, arsenic and
antimony have low tensile strength.
◦
Malleability: Metals can be hammered
into very thin sheets. This tendency of metals is called malleability.
◦
Use: Aluminum makes use of
this property to transform into silvery foils.
◦
Ductility: Metals can be drawn into
thin wires. This property of metals is called ductility.
◦
Use: copper wires.
◦
Conductivity: Metals are good
conductors of heat and electricity. Silver and copper are very good conductors
of electricity.
◦
Exception: Bismuth and tungsten are
poor conductors.
◦
Sonorous: On being hit, metals
produce a typical sound. Hence, they are said to be sonorous.
◦
Use: This property is being
made used in making temple bells.
11. Physical Properties Of
Non-Metals

◦
Physical state:
Non-metals occur as solids, liquids or gases at
normal temperature.
◦
For
example sulphur, phosphorus occurs in solid state while bromine occurs in
liquid state. Gases like oxygen, nitrogen, etc., occur in the gaseous state.
◦
Hardness:
Non-metals are generally
not hard.
◦
Exception:
Diamond (it is a form of
carbon)
◦
Luster:
Non-metals have a dull
appearance.
◦
Exception:
Graphite and iodine are
exceptions as they are shiny and lustrous.
◦
Density:
Non-Metals are generally
soft and have low densities.
◦
Exception:
Diamond is the hardest
naturally occurring substance.
◦
Melting point and boiling point:
Non-metals have low melting point and boiling
point.
◦
Exceptions: carbon
◦
Tensile strength:
Non-metals do not have tensile strength.
◦ Exception:
Carbon fibre (a form of
carbon) is as tensile as steel.
◦ Malleability:
Non-metals
are non-malleable. If hammered, they form a powdery mass. That is, non-metals
in solid state are brittle in nature.
◦ Ductility:
Non-metals are not
ductile.
◦ Exception:
Carbon
fibre is highly ductile.
◦ Conductivity:
Non-Metals
are generally bad conductor of electricity.
◦ Exception:
Graphite
◦ Sonorous:
Non-Metals
do not produce sound (non-sonorous) when hit.
12. Uses
of Metal
◦ Iron is used for making bridges, engine parts, iron-sheet
and bars.
◦ Copper is used for making electrical wires, coins and
statue.
◦ Silver and gold are used for making jewels, in decorative
purposes and photography.
◦ Mercury is used in thermometers and barometers because of
its high density and uniform expansion at different temperature.
◦ Aluminium is used in electrical wires, cables and in
aerospace industries.
◦ Lead is used in automobile batteries, X-ray machines.

13. Uses
of Non-Metals
◦ Diamond (a form of carbon) is used for making jewels,
cutting and grinding equipment.
◦ Graphite is used in making pencil lead.
◦ Sulfur is used in the manufacturing of gun powder and
vulcanization of rubber.
◦ Phosphorus is used in matches, rat poison etc.
◦ Nitrogen is used for manufacturing ammonia.
◦ Chlorine is used as a bleaching agent and in sterilizing
water.
◦ Hydrogen is used as a rocket fuel and hydrogen flame is used
for cutting and welding purposes, as well as a reducing agent

14. The
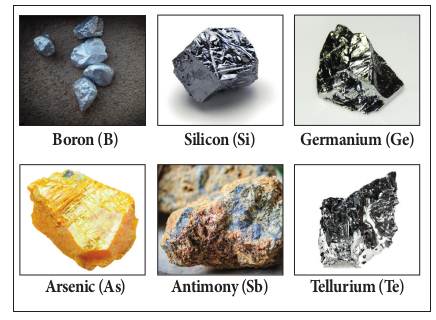
elements which exhibit the properties of metals as well as non-metals are
called metalloids. Examples: boron, silicon, arsenic, germanium, antimony,
tellurium and polonium.
15. Physical properties of
metalloids
·
Metalloids are all solid at room temperature.
·
They can form alloys with other metals
· Some
metalloids, such as silicon and germanium, can act as electrical conductors under
the specific conditions, thus they are called semiconductors.
· Silicon
for example appears lustrous, but is not malleable nor ductile (it is brittle -
a characteristic of some non metals). It is a much poorer conductor of heat and
electricity than the metals
·
The physical properties of metalloids tend to be
metallic, but their chemical properties tend to be non-metallic.
16. Uses of metalloids
◦ Silicon
is used in electronic devices.
◦ Boron
is used in fireworks and as a fuel for ignition in rocket.
17. A compound is a pure
substance which is formed due to the chemical combination of two or more
elements in a fixed ratio by mass.
18. The properties of a
compound are different from those of its constituents.
19. Classification compound based
on the origin of chemical constituents, compounds are classified as inorganic
compounds and organic compounds.
◦ Inorganic
compounds Compounds obtained from nonliving sources such as rock, minerals
etc., are called inorganic compounds. Example: chalk, baking powder etc.,
◦ Organic
compounds Compounds obtained from living sources such as plants, animals etc.,
are called organic compound. Example: Protein, carbohydrates, etc.
20. Both inorganic and
organic compounds exists in all three states of matter I.e., solids, liquids
and gases.
21. Examples of compounds in
solids state
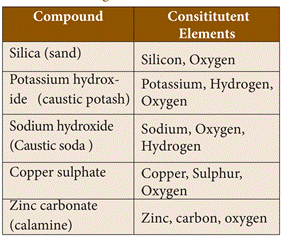
22. Examples
of compounds in liquids state
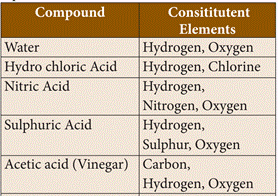
23. Examples
of compounds in gaseous state
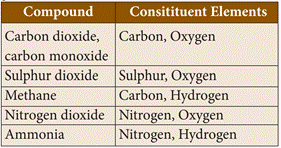

24. Some
useful compounds