Heat
· All
substances around us are made up of matter.
· This
matter is made up of atoms and molecules
· These
atoms vibrate since they are in vibratory motion.
· These
vibrations produce heat energy.
· It is
unidirectional i.e. heat flows from hotter region to colder region.
· When
heat is provided it increases the energy of atoms leading to increase in
vibrations.
· These
vibrations of one atom in a substances sets the adjacent atom in a vibratory
motion.
· Thus
heat energy is transferred from one place to substance via vibrations.
Effects of heat:
- There
are three main changes in the substance when heat is applied.
Expansion:
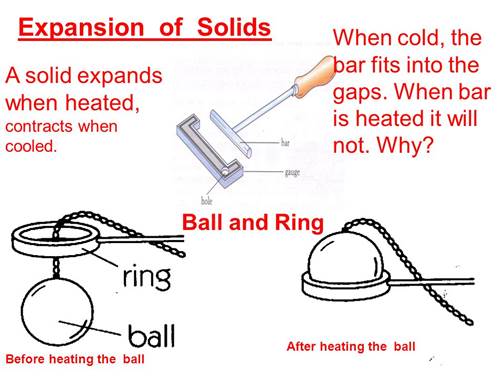
ü When
the ball is heated the atoms in the ball gain heat energy.
ü They
start vibrating and force each other apart.
ü As a
result an expansion takes place.
ü Thus the
ball expands which does not allow the ball to pass through the hole.
ü During
the course of time the ball cools down and the ball is back to its original
shape.
ü This
demonstrates that the expansion takes place in solids.
ü Expansion
also occurs in liquids and gases.
ü The
maximum expansion takes place in gases.
Rise in temperature:
ü When water is heated, water molecules receive
heat energy.
ü We know that as heat is applied it increases
the kinetic energy of the molecules.
ü Increase in energy leads to the increase in
temperature.
ü Thus heat energy causes increase in
temperature.
Change of state:
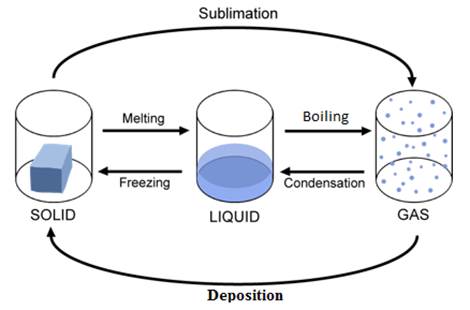
ü Ice
cubes are solid in nature which means the force of attraction in the molecules
is much greater than in water molecules.
ü When we
heat them the force of attraction decreases and the ice cubes convert into
water leading to the transformation in state.
ü Similarly
when we heat water the fore of attraction decreases and it turns into vapor.
ü As
water vapor is gaseous in nature it escapes into the surrounding.
ü Thus
the water level decreases.
ü Heat
energy applied or taken changes the state of matter.
1.
Solid
to liquid (melting)
2.
Liquid
to gas (vaporization)
3.
Solid
to gas (sublimation)
4.
Gas to
liquid (condensation)
5.
Liquid
to solid (freezing)
6.
Gas to
solid (deposition)
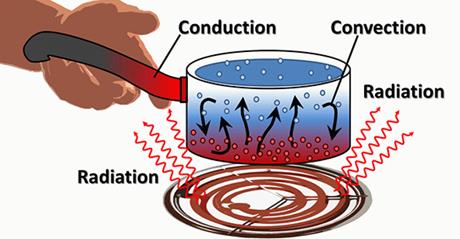
Transfer of heat:
- When heat energy is applied to a substance it
transfers from one part to another. This transfer takes place in any of the
three ways
a)
Conduction
b)
Convection
c)
Radiation
Conduction:
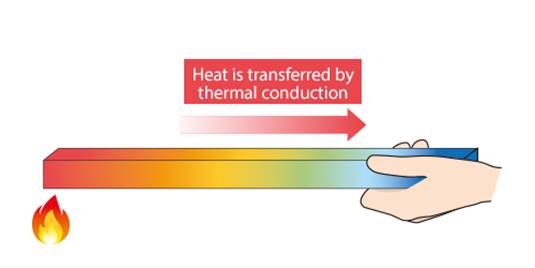
ü When we touch a spoon which was held over a
burner it feels hot. Whereas if we touch wood over a burner it turns into
ashes.
ü Thus metals are good conductors of heat i.e.
they allow the transfer of heat from one place to another.
ü But nonmetals like carbon, phosphorous are bad
conductors of heat as they do not allow the transfer of heat from one to
another.
ü We use conduction in daily life like for
ironing our clothes, handles of utensils are made up of wood or plastic etc.
ü This transfer of heat only occurs in solids.
Convection:
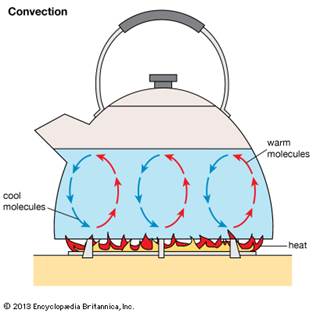
ü When
water in a vessel is heated the water molecules at the bottom of the vessel
heat up first.
ü As they
receive energy they become more active and lighter compared to the other
molecules of water on top.
ü Thus
these lighter energized water molecules move to the top of the water level i.e.
towards the surface.
ü Leading
to the top, molecule to sink down as it is less active.
ü The
above steps are followed leading to a convection transfer of heat energy.
ü This is
how the hot air is lighter and stays at the top
ü Convection
in daily life is the raising of hot air balloon, the flowing of cool air from
colder to a hotter region, etc.
ü This
type of transfer of heat only occurs in liquids and gases.
Radiation:
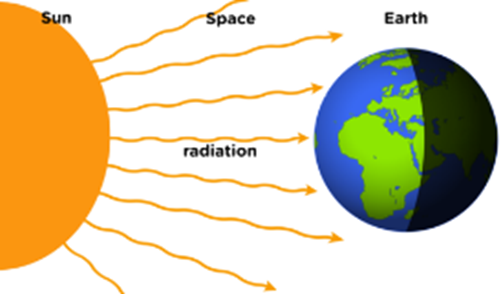
ü It is defined
as the way of heat transfer from one to another in the form of electromagnetic
waves.
ü Heat
energy from the sun reaches the earth in this form.
ü This
type of transfer of heat only occurs in vacuum i.e. in space
ü Radiation
is used in everyday life as we are advised to wear white clothes in summer as
white reflects radiation.
Calorimetry:-
ü Heat
energy along with physical changes also brings about chemical changes.
ü In
order to know about the chemical and physical changes produced we need to know
the amount of heat energy involved.
ü The
technique used to measure the amount of heat involved in a physical or chemical
process is known as calorimetry.
Temperature: It is
a physical quantity which expresses whether an object is hot or cold.
It is measured with the help of
thermometer and has three scales to measure it
a. Celsius
scale
b. Kelvin
scale
c. Fahrenheit
scale
ü Among
them Kelvin scale is most commonly used.
ü Unit of
heat
ü Heat is
a form of energy
ü The
unit of energy in SI unit is joule
ü So heat
is also measured in joule
ü Represented
by J.
ü Although
most commonly used unit is calorie
ü 1
calorie=4.0186J
ü One
calorie- It is the amount of heat energy required to raise the temperature of 1
gram of water through 1 degree Celsius.
Heat capacity:
The amount of heat energy
gained or lost by a substance is determined by three factors
a. Mass of
the substance
b. Change
in temperature of the substance
c. Nature
of the material of the substance
ü Different
substances require different amount of heat energy to reach a particular
temperature this is called as heat capacity.
ü It is
defined as the amount of heat energy required by a substance to raise its
temperature by 1K
ü And is
denoted by C
![]() Heat
capacity= amount of heat energy required (Q)
Heat
capacity= amount of heat energy required (Q)
![]() Raise in temperature ( T)
Raise in temperature ( T)
![]() C’=
Q/ T
C’=
Q/ T
ü The unit of heat capacity is J/K.
Specific heat
capacity:
It is defined as the amount of
heat energy required to raise the temperature of 1 kilogram of a substance by
1K.
![]() Specific
heat capacity = amount of heat energy required (Q)
Specific
heat capacity = amount of heat energy required (Q)
Mass X raise in temperature
![]() C=Q /m X T
C=Q /m X T
The SI unit of specific heat capacity is J/Kg K.
Calorimeter:-
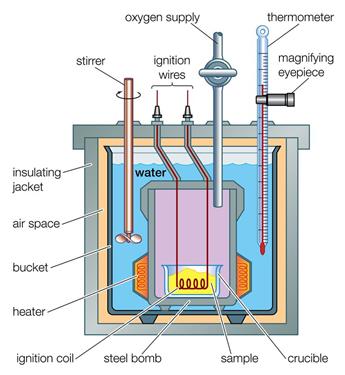
A
colorimeter is a device used to measure the amount of heat gained or lost by a substance .
Construction:
ü It
consists of a vessel made up of metals like copper aluminum which are good
conductor of heat and electricity
ü The
metallic vessel is kept in an insulating jacket to prevent heat loss through
environment
ü There
are two holes in it.
ü Through
one hole a thermometer is inserted to measure the temperature of the contents
ü A
stirrer is inserted through another hole for stirring the contents
ü The
vessel is filled with liquid which is heated by passing currents through
heating element.
Thermostat:-

ü A
thermostat is a device which maintains the temperature of a place or an object
constant.
ü It
turns an appliance or circuit on or off when a particular temperature is
reached
ü The
thermostat may function as a sensor and the controller of a thermal system.
ü The
devices which uses thermostat are water heater, air conditioner, etc.
Thermos flask (vacuum flask):-
ü It
is an insulating storage vessel made to keep its storage hotter or cooler than
the surrounding for a longer time
ü It
is primarily meant to enhance the storage period of a liquid by maintaining a
uniform temperature
ü This
avoids possibilities of bad taste and also the liquid becomes less perishable
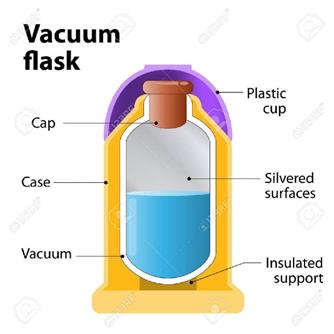
Working:
ü A thermos flask has double walls in
which vacuum is created.
ü It is painted with silver on the
inside
ü The vacuum between the two walls
prevent heat being transferred from the inside to the outside by conduction and
convection
ü With very little air between the
walls there is almost no transfer of heat from then inner wall to the outer
wall or vice versa.
ü Conduction occurs at only two points
where the two walls meet at the top of the bottle and through an insulated
support at the bottom.
ü The silvered walls reflect and
radiate heat back to the liquid in the bottle