Metabolism of Nitrogen
Nitrogen Cycle:
Apart
from carbon, hydrogen, and oxygen,
nitrogen is the most prevalent element in living organisms. Nitrogen is a
constituent of amino acids, proteins, hormones, chlorophylls and many of the vitamins.
Plants compete with microbes for the limited nitrogen that is available in the soil. Thus, nitrogen is a limiting nutrient for
both natural and agricultural eco-systems. Nitrogen exists as two nitrogen
atoms joined by a very strong triple covalent bond (N º N). The
process of conversion of nitrogen (N2) to ammonia is termed as nitrogen fixation.
In
nature, lightning and ultraviolet radiation provide enough energy to convert
nitrogen to nitrogen oxides (NO, NO2, N2O). Industrial
combustions, forest fires, automobile exhausts and power-generating stations
are also sources of atmospheric nitrogen oxides. Decomposition of organic
nitrogen of dead plants and animals into ammonia is called ammonification. Some
of this ammonia volatilises and re-enters the atmosphere but most of it is
converted into nitrate by soil bacteria in the following steps:
2NH3
+ 3O2 → 2N![]() + 2H+ + 2H2O ------ (i)
+ 2H+ + 2H2O ------ (i)
2N![]() + O2 → 2N
+ O2 → 2N![]() ------
(ii)
------
(ii)
Ammonia
is first oxidised to nitrite by the bacteria Nitrosomonas
and/or Nitrococcus. The nitrite is further oxidised
to nitrate with the help of the bacterium Nitrobacter.
These steps are called nitrification.
These nitrifying bacteria are chemoautotrophs.
Ammonia is transported in three forms by ammonia transporters.
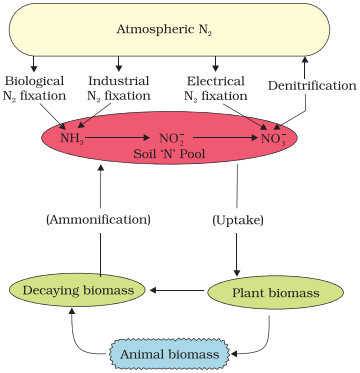
The
nitrogen cycle showing relationship between the three main nitrogen pools – atmospheric
soil, and biomass
The
nitrate thus formed is absorbed by plants and is transported to the leaves. In
leaves, it is reduced to form ammonia that finally forms the amine group of
amino acids. The medium around plant roots turns alkaline on nitrate uptake.
Therefore, nitrate uptake is always accompanied by cation uptake or anion
removal to maintain ionic balance. Nitrate present in the soil is also reduced
to nitrogen by the process of denitrification. It is carried by bacteria
Pseudomonas and Thiobacillus.
Biological
Nitrogen Fixation:
Very few
living organisms can utilise the nitrogen in the form N2, available
abundantly in the air. Only certain prokaryotic species are capable of fixing
nitrogen. Reduction of nitrogen to ammonia by living organisms is called biological nitrogen fixation. The
enzyme, nitrogenase which is capable of nitrogen
reduction is present exclusively in prokaryotes. Such microbes are called N2-
fixers.
N º N ![]() NH3
NH3
The
nitrogen-fixing microbes could be free-living or symbiotic. Examples of
free-living nitrogen-fixing aerobic microbes are Azotobacter
and Beijernickia while Rhodospirillum
is anaerobic and Bacillus free-living. In addition, a number of cyanobacteria
such as Anabaena and Nostoc are also free-living
nitrogen-fixers. Anabaena is a blue-green alga composed of barrel-shapped cells held in a gelatinous matrix. So, it can fix
atmospheric nitrogen.
Symbiotic
Biological Nitrogen Fixation:
Several
types of symbiotic biological nitrogen fixing associations are known. The most
prominent among them is the legume-bacteria relationship. Species of rod-shaped
Rhizobium has such relationship with the roots of several legumes such as alfalfa,
sweet clover, sweet pea, lentils, garden pea, broad bean, clover beans, etc.
The most common association on roots is as nodules. These nodules are small
outgrowths on the roots. The microbe, Frankia, also produces nitrogen-fixing
nodules on the roots of non-leguminous plants (e.g., Alnus).
Both Rhizobium and Frankia are free-living organisms present in the soil, but as symbionts can fix atmospheric
nitrogen.
Uproot
any one plant of a common pulse, just before flowering. There will be
near-spherical outgrowths on the roots. These are nodules. If you cut through
them you will notice that the central portion is red or pink. The nodules are in pink due to the presence of leguminous
haemoglobin or leg-haemoglobin.
Nodule
Formation:
Nodule
formation involves a sequence of multiple interactions between rhizobium and
roots of the host plant. Principal stages in the nodule formation are
summarised as follows:
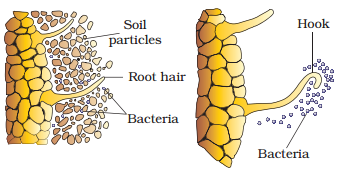
Rhizobia
multiply and colonise the surroundings of roots and get attached to epidermal
and root hair cells. The root-hairs curl and the bacteria invade the root-hair.
An infection thread is produced carrying the bacteria into the cortex of the
root, where they initiate the nodule formation in the cortex of the root. Then
the bacteria are released from the thread into the cells which leads to the
differentiation of specialised nitrogen fixing cells. The nodule thus formed,
establishes a direct vascular connection with the host for the exchange of nutrients. These events are
depicted in below figure.
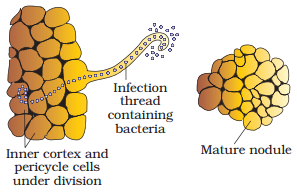
Development
of root nodules in soyabean : (1) Rhizobium bacteria contact a susceptible root hair,
divide near it, (2) Successful infection of the root hair causes it to curl,
(3) Infected thread carries the bacteria to the inner cortex. The bacteria get
modified into rod-shaped bacteroids and cause inner
cortical and pericycle cells to divide. Division and
growth of cortical and pericycle cells lead to nodule
formation, (4) A mature nodule is complete with
vascular tissues continuous with those of the root.
The
nodule contains all the necessary biochemical components, such as the enzyme nitrogenase and leghaemoglobin. Leghaemoglobin is the
haemoglobin like red pigments found in the root nodules of legumes and reported
to function as an oxygen-carrying pigment in symbiotic nitrogen fixation. The
enzyme nitrogenase is a Mo-Fe protein and catalyses
the conversion of atmospheric nitrogen to ammonia, the first stable product of
nitrogen fixation.
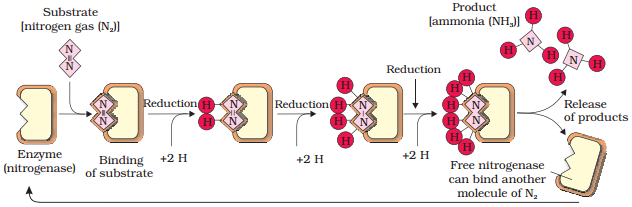
Steps
of conversion of atmospheric nitrogen to ammonia by nitrogenase
enzyme complex found in nitrogen-fixing bacteria
The reaction is as follows:
N2
+ 8e− + 8H+ + 16ATP → 2NH3 + H2
+ 16ADP + 16Pi
The
enzyme nitrogenase is highly sensitive to the
molecular oxygen; it requires anaerobic conditions. The nodules have adaptations
that ensure that the enzyme is protected from oxygen. To protect these enzymes,
the nodule contains an oxygen scavenger called leg-haemoglobin. It is
interesting to note that these microbes live as aerobes under free-living
conditions (where nitrogenase is not operational),
but during nitrogen-fixing events, they become anaerobic (thus protecting the nitrogenase enzyme). From above reaction that the ammonia
synthesis by nitrogenease requires a very high input
of energy (8 ATP for each NH3 produced). The energy required, thus,
is obtained from the respiration of the host cells.
The fate of Ammonia:
At
physiological pH, the ammonia is protonated to form N![]() (ammonium) ion. While most of the plants can
assimilate nitrate as well as ammonium ions, the latter is quite toxic to
plants and hence cannot accumulate in them. There are two main ways in which
the N
(ammonium) ion. While most of the plants can
assimilate nitrate as well as ammonium ions, the latter is quite toxic to
plants and hence cannot accumulate in them. There are two main ways in which
the N![]() is used to synthesise amino acids in plants:
is used to synthesise amino acids in plants:
i.
Reductive
Amination: In these processes, ammonia reacts with a-ketoglutaric
acid and forms glutamic acid as indicated in the equation given below :
![]()
ii.
Transamination: It
involves the transfer of an amino group
from one amino acid to the keto group of a keto acid. Glutamic acid is the main
amino acid from which the transfer of NH2, the amino group takes
place and other amino acids are formed through transamination. The enzyme
transaminase catalyses all such reactions. For example,

The two
most important amides – asparagine and glutamine – found in plants are a
structural part of proteins. They are formed from two amino acids, namely
aspartic acid and glutamic acid, respectively, by addition of another amino
group to each. The hydroxyl part of the acid is replaced by another NH2
– radicle. Since amides contain more nitrogen than the amino acids, they are
transported to other parts of the plant via xylem vessels. In addition, along
with the transpiration stream the nodules of some plants (e.g., soyabean) export the fixed nitrogen as ureides.
These compounds also have a particularly high nitrogen to carbon ratio.




