Alkanes
Alkanes
are saturated open chain hydrocarbons containing carbon - carbon single bonds.
Methane (CH4) is the first member of this family. Methane is a gas
found in coal mines and marshy places. The hydrocarbon with molecular formula C2H6
known as ethane. Thus you can consider C2H6 as derived
from CH4 by replacing one hydrogen atom by –CH3 group.
The next molecules will be C3H8 C4H10…
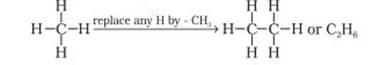
These hydrocarbons are inert under
normal conditions as they do not react with acids, bases and other reagents.
Hence, they were earlier known as paraffins. In
alkanes, tetrahedra are joined together in which C-C
and C-H bond lengths are 154 pm and 112 pm respectively.
Uses of Alkanes
Alkanes
are very versatile and are being used as solvents, heating oils, fuels,
in fat synthesis, in the synthesis of fatty acids by air oxidation, in the
manufacture of albumen, in the transformation to olefins, etc. The
latter are starting materials for the preparation of alkyl benzenes which play
an important role in the synthesis of degradable detergents.
Methane:
Methane
in form of natural gas is used for running scooters, cars, buses, etc. LPG is
used as a fuel in homes as well as in industry. Methane plays an important
role in the generation of a carbon dioxide-hydrogen mixture either by
incomplete combustion of methane and/or by reaction of the alkane with water
vapor. It is used for manufacture of halogen containing compounds such as CH2Cl2,
CHCL3 etc. which are used as solvents
both in laboratory and industry.
Catalytic
oxidation of alkanes gives alcohols, aldehydes and carboxylic acids.
Higher
alkanes in form gasoline, kerosene oil, diesel, lubricating oils and paraffin
wax are widely used.