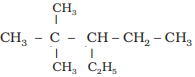Nomenclature and
Isomerism
The
compound having same molecular formula but different physical and chemical are
called as isomers and this phenomenon is called Isomerism.
Compounds
which have same molecular formula, but their structural formulas are different,
are called structural isomers.
First
three alkanes – methane, ethane and propane have only one structure but higher
alkanes can have more than one structure.
Four
carbon atoms of C4H10 can be joined either in a
continuous chain or with a branched chain in the following two ways:
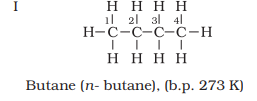
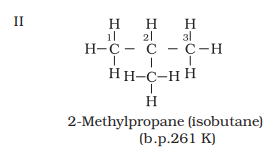
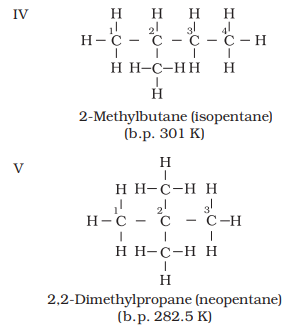
Structures I and II
possess same molecular formula but differ in their boiling points and other
properties.
Similarly
structures III, IV and V possess the same molecular formula but have different
properties. Structures I and II are isomers of butane, whereas structures III,
IV and V are isomers of pentane. Since difference in properties is due to
difference in their structures, they are known as structural isomers. It is
also clear that structures I and III have continuous chain of carbon atoms but
structures II, IV and V have a branched chain. Such structural isomers which
differ in chain of carbon atoms are known as chain isomers. Thus, you have seen
that C4H10 and C5H12 have two and
three chain isomers respectively.
·
Based upon the number of carbon
atoms attached to a carbon atom, the carbon atom is termed as -
o Primary
(1°),
o Secondary
(2°),
o Tertiary
(3°) or
o Quaternary
(4°).
·
Carbon atom attached to no other
carbon atom as in methane or to only one carbon atom as in ethane is called
primary carbon atom.
·
Terminal carbon atoms are always
primary.
·
Carbon atom attached to two
carbon atoms is known as secondary.
·
Tertiary carbon is attached to
three carbon atoms and neo or quaternary carbon is attached to four carbon
atoms.
Nomenclature of few organic
compounds
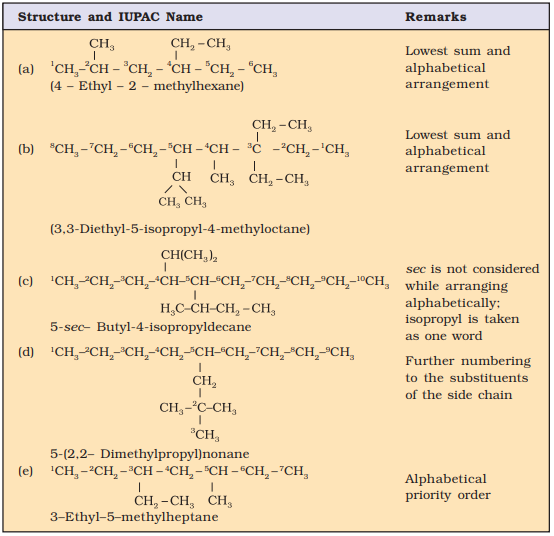
If it is important to
write the correct IUPAC name for a given structure, it is equally important to
write the correct structure from the given IUPAC name. To do this, first of
all, the longest chain of carbon atoms corresponding to the parent alkane is
written. Then after numbering it, the substituents are attached to the correct
carbon atoms and finally valence of each carbon atom is satisfied by putting
the correct number of hydrogen atoms.
Example: Structure of
3-ethyl-2, 2–dimethylpentane
i)
Draw the chain of five carbon atoms:
C
– C – C – C – C
ii) Give number to carbon
atoms:
C1
– C2 – C3 – C4 – C5
iii) Attach ethyl group at
carbon 3 and two methyl groups at carbon 2
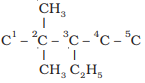
iv) Satisfy the valence of
each carbon atom by putting requisite number of hydrogen atoms: