Chemical Properties
Alkanes are
saturated hydrocarbons. They contain only strong C-C and C-H sigma bonds.
Hence, alkanes are quit less reactive and are known as paraffins (parum-little
affinis-reactivity or affinity). Alkanes are generally in towards acids, bases,
oxidizing and reducing agents. However, they undergo the following
reactions under certain conditions.
Substitution Reactions
Ø One or
more hydrogen atoms of alkanes can be replaced by halogens, nitro group and
sulphonic acid group.
Ø Halogenation
takes place either at higher temperature (573-773 K) or in the presence of
diffused sunlight or ultraviolet light.
Ø Lower
alkanes do not undergo nitration and sulphonation reactions.
These
reactions in which hydrogen atoms of alkanes are substituted are known as
substitution reactions. As an example, chlorination of methane is given below:
Halogenation
![]()
![]()
![]()

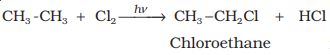
It is
found that the rate of reaction of alkanes with halogens is F2 >
Cl2 > Br2 > I2. Rate of replacement of
hydrogens of alkanes is : 3° > 2° > 1°. Fluorination is too violent to be
controlled. Iodination is very slow and a reversible reaction. It can be
carried out in the presence of oxidizing agents like HIO3 or HNO3.
![]()
![]()
Halogenation
is supposed to proceed via free radical chain mechanism involving three steps
namely initiation, propagation and termination as given below:
Mechanism
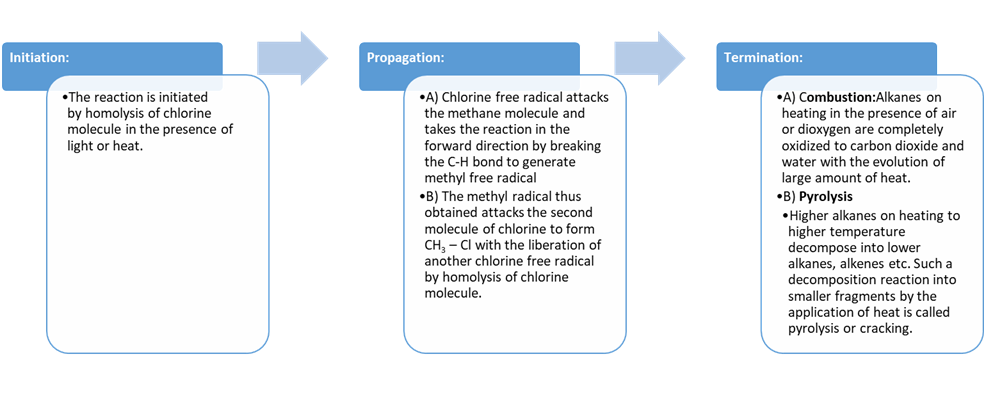
(i) Initiation:
The reaction is initiated by homolysis of chlorine molecule
in the presence of light or heat. The Cl–Cl bond is weaker than the C–C and C–H
bond and hence, is easiest to break.
![]()
(ii) Propagation:
Chlorine free radical attacks
the methane molecule and takes the reaction in the forward direction by
breaking the C-H bond to generate methyl free radical with the formation of
H-Cl.
![]()
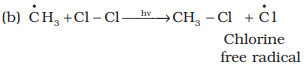
The methyl radical thus obtained attacks the second molecule
of chlorine to form CH3 – Cl with the liberation of another chlorine
free radical by homolysis of chlorine molecule.
The chlorine and methyl free radicals generated above repeat
steps (a) and (b) respectively and thereby setup a chain of reactions. The
propagation steps (a) and (b) are those which directly give principal products,
but many other propagation steps are possible and may occur. Two such steps
given below explain how more highly haloginated products are formed.
![]()
![]()
(iii) Termination:
The reaction stops after some
time due to consumption of reactants and/or due to the following side
reactions:
The possible chain terminating steps are:
![]()
![]()
![]()
Though in (c), CH3 –
Cl, the one of the products is formed but free radicals are consumed and the
chain is terminated. The above mechanism helps to understand the reason for the
formation of ethane as a byproduct during chlorination of methane.
Combustion
Alkanes on heating in the
presence of air or dioxygen are completely oxidized to carbon dioxide and water
with the evolution of large amount of heat.
![]()
![]()
The general combustion equation for any alkane is:
![]()
Due to the evolution of large amount of heat during
combustion, alkanes are used as fuels.
During incomplete combustion of alkanes with insufficient
amount of air or dioxygen, carbon black is formed which is used in the
manufacture of ink, printer ink, black pigments and as filters.
![]()
Pyrolysis
Higher alkanes on heating to higher temperature decompose
into lower alkanes, alkenes etc. Such a decomposition reaction into smaller
fragments by the application of heat is called pyrolysis or cracking.
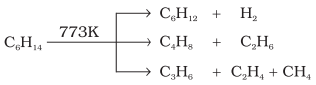
Pyrolysis of alkanes is believed to be a free radical
reaction. Preparation of oil gas or petrol gas from kerosene oil or petrol
involves the principle of pyrolysis.
For example, dodecane, a constituent of kerosene oil on
heating to 973K in the presence of platinum, palladium or nickel gives a
mixture of heptane and pentene.
![]()