Preparation
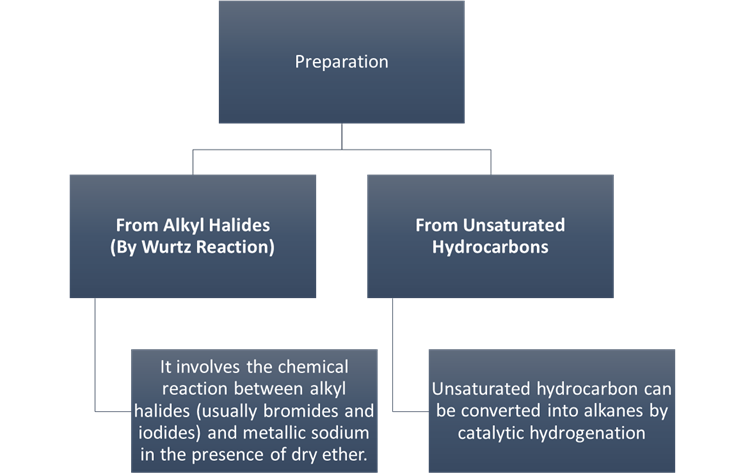
From Alkyl Halides (By Wurtz
Reaction)
Alkyl halides are
halogen derivatives of alkanes with general formula R-X. They can be used to
prepare alkanes by Wurtz reaction:
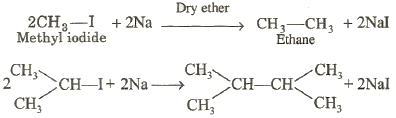
It involves the chemical
reaction between alkyl halides (usually bromides and iodides) and metallic
sodium in the presence of dry ether. The product is the symmetrical alkane
containing twice the number of carbon atoms present in the alkyl halide.
![]()
For examples
From
Unsaturated Hydrocarbons
Unsaturated
hydrocarbon can be converted into alkanes by catalytic hydrogenation. The
catalyst used may be nickel, platinum or palladium. These metals absorb
dihydrogen gas on their surface and weaken the hydrogen-hydrogen bond. Platinum
and palladium catalyst the reaction at room temperature while nickel requires
higher temperature and pressure. The reaction is called Sebatier
and Senderenís reaction.
![]()
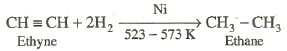
The process of addition of hydrogen to an unsaturated compound
in presence of a catalyst is called hydrogeneration or reduction.