Chemical Properties
Addition of hydrogen
Hydrogenation is the addition of hydrogen to an alkene.
Although this reaction is exothermic, it is very slow. The addition of a metal catalyst,
such as platinum, palladium, nickel, or rhodium, greatly increases the reaction
rate. Although this reaction seems simple, it is a highly complex
addition.
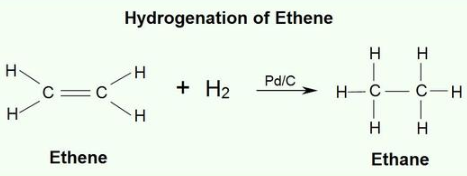
An example of an alkene
addition reaction is a process called hydrogenation.
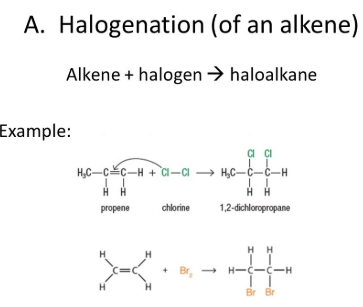
Addition of halogens
A halogen addition
reaction is a simple organic reaction where
a halogen molecule is added to the carbon–carbon double
bond of an alkene functional group.
The general chemical
formula of the halogen addition reaction is:
C=C + X2 →
X−C−C−X
(X represents
the halogens bromine or chlorine, and in this case, a
solvent could be CH2Cl2 or CCl4).
The product is a vicinal dihalide.
This type of
reaction is a halogenation and an electrophilic addition.
The
halogens Br2 and Cl2 add to alkenes. This may
be surprising because it is not immediately apparent that an electrophile—which
is necessary to start an electrophilic addition reaction—is present.
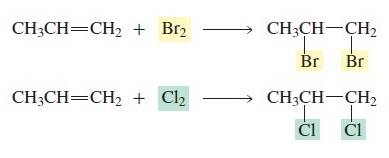
Addition of hydrogen halides
Hydrogen halides (HCl, HBr, HI) add up to alkenes
to form alkyl halides. The order of reactivity of the hydrogen halides is HI
> HBr > HCl. Like
addition of halogens to alkenes, addition of hydrogen halides is also an
example of electrophilic addition reaction.
Addition reaction of HBr to
symmetrical alkenes
Addition reactions of HBr
to symmetrical alkenes (similar groups attached to double bond) take place by
electrophilic addition mechanism.
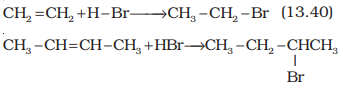
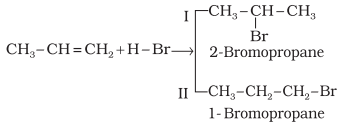
Addition reaction of HBr to unsymmetrical alkenes (Markovnikov Rule)
How will H – Br add to propene? The two
possible products are I and II.
Markovnikov,
a Russian chemist made a generalisation in 1869 after
studying such reactions in detail. These generalisations
led Markovnikov to frame a rule called Markovnikov rule.
The rule
states that negative part of the addendum (adding molecule) gets attached to
that carbon atom which possesses lesser number of hydrogen atoms.
Thus
according to this rule, product I i.e., 2-bromopropane is expected. In actual
practice, this is the principal product of the reaction. This generalisation of Markovnikov rule can be better understood
in terms of mechanism of the reaction.
Mechanism
Hydrogen bromide provides
an electrophile, H+, which attacks the double bond to form
carbocation as shown below :
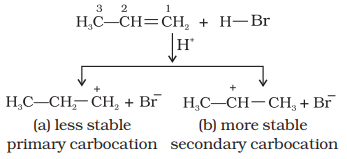
(i) The secondary
carbocation (b) is more stable than the primary carbocation (a), therefore, the
former predominates because it is formed at a faster rate.
(ii) The carbocation (b) is attacked by Br–
ion to form the product as follows :
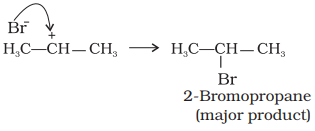
Anti Markovnikov addition or
peroxide effect or Kharash effect
In the
presence of peroxide, addition of HBr to
unsymmetrical alkenes like propene takes place contrary to the Markovnikov
rule. This happens only with HBr but not with HCl and Hl. This addition reaction was observed by M.S. Kharash and F.R. Mayo in 1933 at the University of Chicago.
This reaction is known as peroxide or Kharash effect
or addition reaction anti to Markovnikov rule.
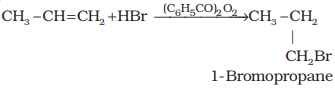
Mechanism:
Peroxide effect proceeds via free radical
chain mechanism as given below:
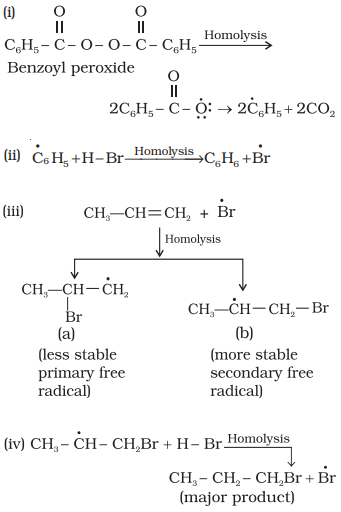
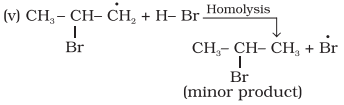
The
secondary free radical obtained in the above mechanism (step iii) is more
stable than the primary. This explains the formation of 1-bromopropane as the
major product. It may be noted that the peroxide effect is not observed in
addition of HCl and HI. This may be due to the fact
that the H–Cl bond being stronger (430.5 kJ mol–1)
than H–Br bond (363.7 kJ mol–1), is not
cleaved by the free radical, whereas the H–I bond is weaker (296.8 kJ mol–1) and iodine free radicals combine to form
iodine molecules instead of adding to the double bond.
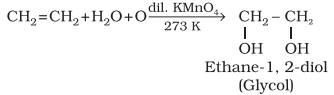
Oxidation:
Alkenes
on reaction with cold, dilute, aqueous solution of potassium permanganate
(Baeyer’s reagent) produce vicinal glycols. Decolorisation
of KMnO4 solution is used as a test for unsaturation.
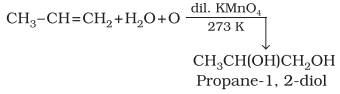
Acidic
potassium permanganate or acidic potassium dichromate oxidises
alkenes to ketones and/or acids depending upon the nature of the alkene and the
experimental conditions.
![]()
![]()
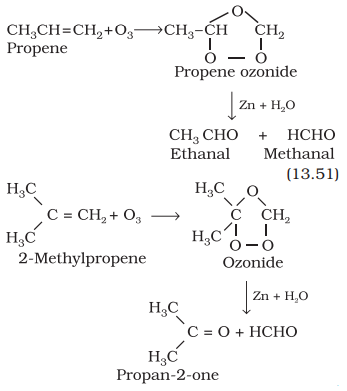
Ozonolysis:
Ozonolysis of
alkenes involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide
by Zn-H2O to smaller molecules. This reaction is highly useful in
detecting the position of the double bond in alkenes or other unsaturated compounds.