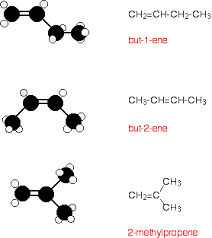
Physical Properties
The melting and boiling points of
alkenes are determined by the regularity of the packing, or the closeness, of
these molecules. Alkene isomers that can achieve more regular packing have higher
melting and boiling points than molecules with the same molecular formula but
weaker dispersion forces. Alkenes are non-polar, and they are both immiscible
in water and less dense than water. They are generally soluble in organic
solvents. In addition, they do not conduct electricity.