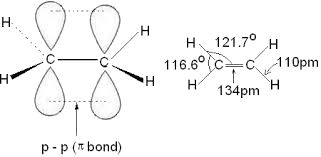
Structure of Double Bond
Carbons in the double bond
of butene are sp2 hybridized. Side on p-p
orbital overlap creates a π-bond. Angles
around the carbons in the double bond are ~ 120º. Thus, all of the atoms bonded
to the sp2 hybridized carbon lie in a plane. Carbon-Carbon double
bond length is ~ 1.34 Å (single bonds in alkane are ~ 1.54 Å.
Alkenes are readily attacked by reagents or compounds which are in
search of electrons. Such reagents are called electrophiles or electrophilic
reagents. Further, the presence of π-bond makes
alkenes less stable and more reactive than alkanes and hence readily change
into compounds containing single bonds by adding electrophilic reagents.
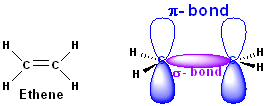
Alkenes are part of an homologous series. They
have the functional group of a C=C (double bond between two
carbons) and the general formula of CnH2n, where n
is the number of carbon molecules.
There
are two types of bonds in alkenes because of the different
electron sub-shells.
·
σ bond (sigma)
·
π bond (pi)
There is a σ bond
(sigma) which is when two p sub
orbitals overlap, and the π bond (pi) is above and below
the atoms, it tends to be weaker because of this and it also has no rotation
which is why isomerism exists. Because of this bonding, the double bond is a
center of high electron density, meaning it is a great place for electrophiles
to attack.