Geometrical Isomerism
Isomers are
compounds that have the same molecular formula, but have different arrangements
of atoms. Isomerism is very important in organic chemistry, as it gives rise to
the millions of organic compounds that exist and, in some ways, helps to
function as a coding mechanism.
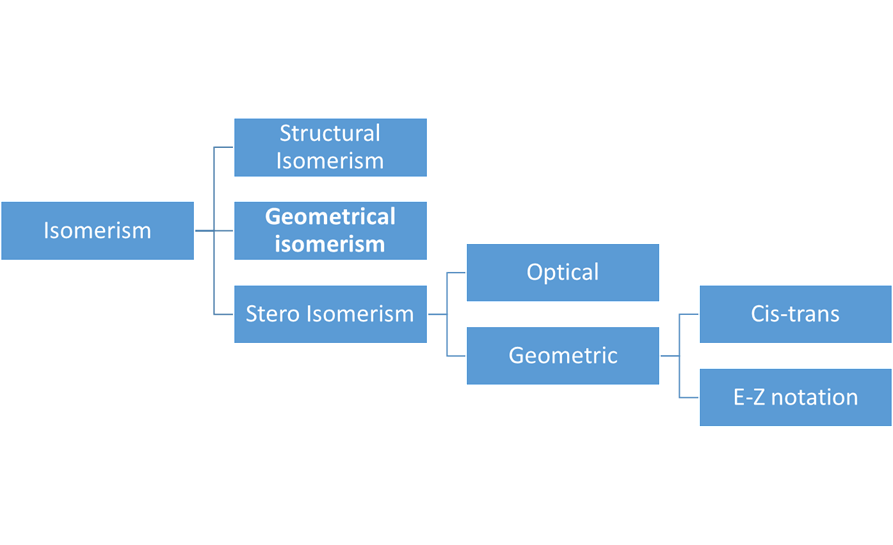
There are two categories of isomerism:
·
Structural
·
Stereisomerism
Structural isomerism:
As in alkanes, ethene (C2H4) and
propene (C3H6) can have only one structure but alkenes
higher than propene have different structures. Alkenes possessing C4H8
as molecular formula can be written in the following three ways:
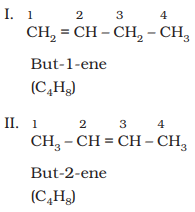
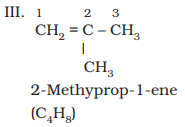
Structures I and III, and II and III are the examples of
chain isomerism whereas structures I and II are position isomers.
Geometrical isomerism:
Doubly bonded carbon atoms have to satisfy the remaining two
valences by joining with two atoms or groups. If the two atoms or groups
attached to each carbon atom are different, they can be represented by YX C = C
XY like structure.
YX C = C XY can be represented in space in the following two
ways :
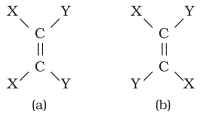
There
are two types of stereoisomerism,
·
Optical
·
Geometric
Optical Isomers
These are isomers which possess a non-superimposable mirror
image. However, we will not be covering these in this course.
Geometric Isomers
This type of isomerism occurs in compounds that have some
restricted rotation. Geometric isomerism is not only a feature of organic
compounds, as inorganic compounds display geometric isomerism. In
geometric isomerism, there must be a least two different groups attached to the
carbon on which there is restricted rotation.
There
are two ways to name geometric isomers,
·
Cis-trans
·
E-Z notation
Cis-Trans
For cis-trans
geometric isomerism to occur, at least one of the groups must appear on both
carbons. If groups that are the same appear on the same side, the isomer is
referred to as ‘cis’ and if they appear on different sides it is ‘trans’.
Examples of cis-trans isomerisms are found below in figures. Notice the
location of the chlorine in below figure, when the chlorine is on the same
side, it is cis and when it is on different sides, as in the image on the
right, it is trans.
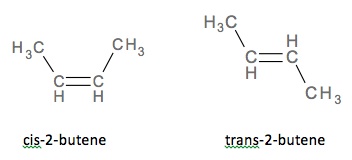
The cis and trans isomers
for 2-butene.
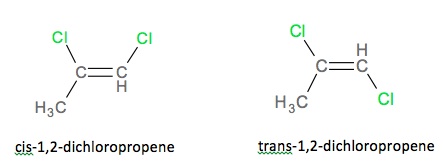
The cis and trans isomers for 1,
2-dichloropropene.
E-Z
Notation
This notation can be applied to all geometric isomers and is
therefore preferred by IUPAC. However, it is a more complicated notation and so
many prefer to use the cis-trans notation, when possible. If we consider the
tri-substituted alkene in the example below, we would recognise that the
cis-trans notation could not be used, although the compound displays geometric
isomerism.
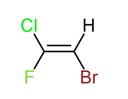
A tri-substituted alkene.
The Cahn-Ingold-Prelog rules are applied to
determine the priority of the groups where is restricted rotation. If the
groups that have the same priority are on opposite sides of the double bond,
then the compound is stated as E. If these groups are the on the same side,
then the compound is stated as Z.