Chemical
Properties
†††††††††††††† Alkynes
are unsaturated hydrocarbons. Like alkenes characteristic reactions of alkynes
are electrophilic addition reactions. However, alkynes are less reactive than
alkenes towards electrophilic addition reactions. Alkynes show acidic nature, addition
reactions and polymerisation reactions as follows:
Acidic
character of alkyne:
†††††††††††††† Sodium metal and sodamide (NaNH2) are strong bases. They react
with ethyne to form sodium acetylide
with the liberation of dihydrogen gas.
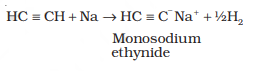
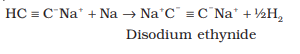
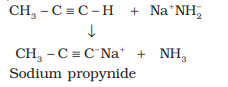
Addition
reactions:
†††††††††††††† Alkynes contain a triple bond, so
they add up, two molecules of dihydrogen, halogen, hydrogen halides etc.
Formation of the addition product takes place according to the following steps.
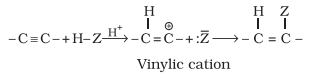
†††††††††††††† The
addition product formed depends upon stability of vinylic
cation. Addition in unsymmetrical alkynes takes place
according to Markovnikov rule.
Addition
of dihydrogen:
†††††††††††††† Alkynes react readily
with hydrogen in the presence of finely-divided nickel, platinum or palladium
as catalysts. The process is known as catalytic hydrogenation. The ultimate
products of this reaction are alkanes.
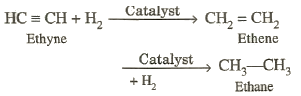
†††††††††††††† The hydrogenation of alkynes can
be restricted to alkenes by employing suitable reaction conditions. For
example, use of Lindlarís can restrict the
hydrogenation to alkene stage. Reduction of alkynes to alkene stage can also be
carried out with sodium or lithium in liquid ammonia.

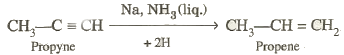
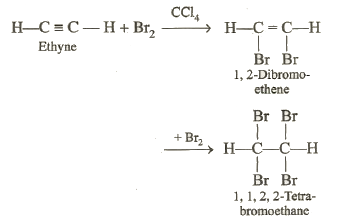
Addition of Halogens:
Halogens, especially chlorine and bromine add on alkynes readily producing a tetra-halogen derivatives. The reaction is carried out in inert solvent like carbon†††† tetrachloride. Due to the presence of two n-bonds, each molecule of the alkyne can react with two molecules of the halogen. For example, the addition of bromine to ethyne can be controlled to give 1, 2-dibromoethene or 1, 1, 2, 2-tetrabromoethane.
Addition
of hydrogen halides:
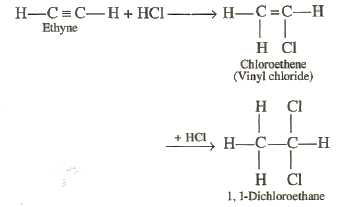
Addition
of water (Hydration):
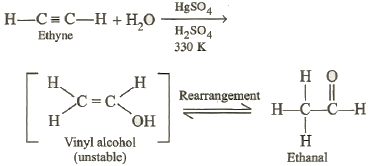
†††††††††††††† Alkynes undergo
hydration in the presence of 60% H2SO4 and mercury
(m tetraoxosulphate (VI) as catalyst at about 330 K.
The products are carbonyl compounds. In case of ethyne,
ethanal is produced. The initially formed vinyl
alcohol is unstable and rearranges to more stable ethanol.
|
Reaction |
Result |
|
Acidic character of alkyne |
liberation of dihydrogen gas |
|
Addition
of dihydrogen |
Alkyne to alkene to alkanes |
|
Addition of Halogens |
tetra-halogen derivatives |
|
Addition
of hydrogen halides |
tetra-halogen
derivatives |
|
Addition
of water (Hydration) |
Carbonyl compounds |