Physical Properties
Properties
The
first three alkynes are gases while those containing five to thirteen carbon
atoms are liquids and higher alkynes are solids. Hybridization
due to triple bonds allows the uniqueness of alkyne structure. This triple bond
contributes to the nonpolar bonding strength, linear, and the acidity of
alkynes.
![]()
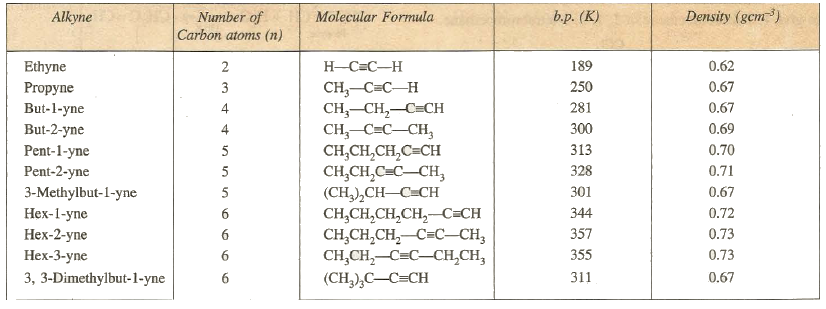
Physical
Properties
·
Physical state, colour,
smell, etc:
The first three members of the family,i.e.,
ethane,propene and butenes
are colourness gases ; the next fourteen members (C5-C18)
are liquid while the higher ones are solids.
·
Solubility:
They are insoluble in water, but are fairly soluble in non-polar solvents such
as beneze,petroleum ether,etc.
·
Boiling points: Their boiling points increases regularly
with increases in molecular mass. On the average, the boiling points generally
increases by 20-30 K for the addition of each CH2 group to the
chain.