Intermolecular forces
Intermolecular forces are attractions between one molecule and a neighbouring molecule. Intermolecular forces are electrostatic in nature; that is, they arise from the interaction between positively and negatively charged species. They are also known as Van der Waals forces named after the Dutch scientist, Johannes van der Waals, who discovered them. They are of three types,
i. Dispersion or London forces
ii. Dipole – dipole forces, and
iii. Dipole – induced dipole forces
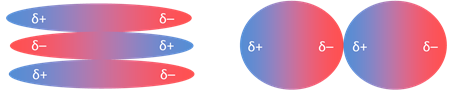
Dipole - Dipole Forces:
Polar molecules have permanent
dipoles with charges (δ) which can be positive (δ+) or
negative (δ−). When two molecules combine they arrange
themselves to get maximum stability. They tend to align themselves so that the
positive end of one dipole is near the negative end of another and vice versa,
the force produced when this happens is called dipole – dipole force.
Dipole-dipole
interaction energy between stationary polar molecules is proportional to
![]() and that between rotating polar molecules is
proportional to
and that between rotating polar molecules is
proportional to ![]() ,
where r is the distance between polar molecules.
,
where r is the distance between polar molecules.
Dispersion or London Forces:
Nonpolar molecules have electrons that are symmetrically distributed around the nucleus. Assuming that the charge becomes unsymmetrical, and then the temporary dipoles get attracted to oppositely charges dipoles of other molecules thus creating a weak force of attraction. This force is called dispersion force or London force as it was discovered by German physicist Fritz London.
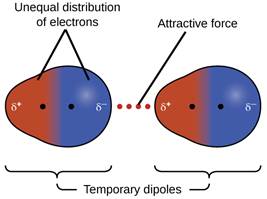
These forces are always attractive and interaction energy is inversely proportional to the sixth power of the distance between two interacting particles, i.e., 1/r 6 where r is the distance between two particles.
Dipole – Induced Dipole
Forces:
This
type of attractive force operates between the polar molecules having permanent
dipole and the molecules lacking permanent dipole. Imagine a molecule which has
a temporary polarity being approached by one which happens to be entirely
non-polar just at that moment. As one molecule approaches, its electrons will
tend to be attracted by the slightly positive end of the other one. This sets
up an induced dipole in the approaching molecule, which is orientated
in such a way that the δ+
end of one is attracted to
the δ− end of the other.
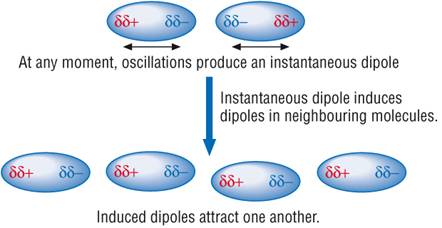
An instant later
the electrons in the first molecule may well have moved up the other end. In
doing so, they will repel the electrons in the right hand one. The polarity of
both molecules reverses, but you still have δ+
attracting δ−.
As long as the molecules stay close to each other the polarities will continue
to fluctuate in synchronisation so that the attraction is always maintained. In this case also interaction energy
is proportional to ![]() where r is the distance between two molecules.
where r is the distance between two molecules.
There is no reason why this has to be restricted to two molecules. As long as the molecules are close together this synchronised movement of the electrons can occur over huge numbers of molecules. This diagram shows how a whole lattice of molecules could be held together in a solid using Van der Waals dispersion forces. An instant later, of course, you would have to draw a quite different arrangement of the distribution of the electrons as they shifted around - but always in synchronisation.
Hydrogen Bonds:
Many elements form compounds with hydrogen. These molecules get larger with more electrons, and so Van der Waals dispersion forces become greater. Especially when hydrogen combines with nitrogen to form NH3, with oxygen to form H2O and with fluorine to form HF, there are some additional intermolecular forces of attraction. These relatively powerful intermolecular forces are described as hydrogen bonds.