Thermal Energy
Thermal energy is the
energy of a body arising from motion of its atoms or molecules. It is directly
proportional to the temperature of the substance. It is the measure of average kinetic
energy of the particles of the matter and is thus responsible for movement of
particles. This movement of particles is called thermal motion.
Intermolecular Forces vs Thermal Energy:
Intermolecular forces
tend to keep the molecules together but thermal energy of the molecules tends
to keep them apart.
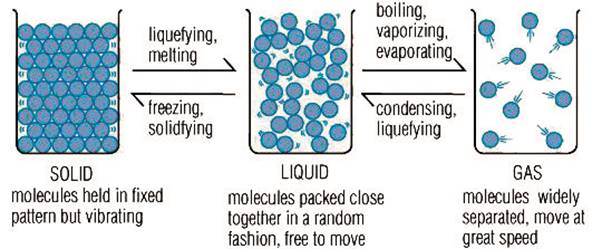
The three states of
matter are the result of balance between the intermolecular forces and the
thermal energy of the molecules. As the temperature is increased the molecules
become larger, the number of electrons increases and the intermolecular forces
become stronger and they start to push away from each other. Thus on applying
thermal energy to a solid it changes to liquid which in turn changes to a gas.
Consequently, a gas on compression changes to a liquid which on further
compression changes to a solid.