Liquefaction of Gases
Thomas Andrews plotted isotherms of carbon dioxide at various
temperatures.
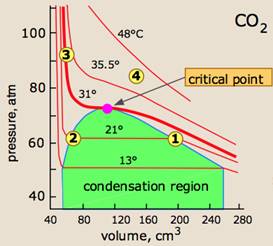
From these he surmised that,
® Real gases behaved like carbon
dioxide.
® Isotherms of carbon dioxide at high
temperatures looked like an ideal gas.
® At lesser temperature the shape of
the curve changes.
® The gas could not be liquefied even
at high temperatures.
® Liquid carbon dioxide first appears
at 31 ͦ C, which is called critical temperature Tc.
® Volume of one mole of gas at this
temperature is called critical volume Vc.
® Pressure at this point is called
critical pressure pc.
® On more pressure being applied at 21
ͦ C the gas changes to liquid at point 1.
® Thus we see that a point like 4
represents gaseous state. A point like 3 represents liquid state and a point 2
or 1 under the dome shaped area represents existence of liquid and gaseous
carbon dioxide in equilibrium.
® All the gases upon compression at
constant temperature (isothermal compression) show the same behaviour as shown
by carbon dioxide.
® Compression brings the molecules in
close vicinity and cooling slows down the movement of molecules therefore,
intermolecular interactions may hold the closely and slowly moving molecules
together and the gas liquefies.
® It is possible to change a gas into
liquid or a liquid into gas by a process in which always a single phase is
present.
Thus there is continuity between the gaseous and liquid state. The term
fluid is used for either a liquid or a gas to recognise this continuity. Thus a
liquid can be viewed as a very dense gas. Liquid and gas can be distinguished
only when the fluid is below its critical temperature and its pressure and
volume lie under the dome, since in that situation liquid and gas are in
equilibrium and a surface separating the two phases is visible. A gas below the
critical temperature can be liquefied by applying pressure, and is called vapour of the substance.
Problem:
Gases possess characteristic critical temperature which depends upon the
magnitude of intermolecular forces between the gas particles. Critical
temperatures of ammonia and carbon dioxide are 405.5 K and 304.10 K
respectively. Which of these gases will liquefy first when you start cooling
from 500 K to their critical temperature?
Solution:
Ammonia will liquefy first because its critical temperature will be
reached first. Liquefaction of CO2 will require more cooling.