Liquid State
® Liquids are denser than gases hence
intermolecular forces are greater in liquid state than gaseous state.
® Liquids have definite volume, can
flow so can be poured and assume the shape of the container they are poured
into.
Vapour Pressure:
® If an evacuated container is partially filled with a liquid, a portion of liquid evaporates to fill the remaining volume of the container with vapour. Initially the liquid evaporates and pressure exerted by vapours on the walls of the container (vapour pressure) increases. After some time it becomes constant, an equilibrium is established between liquid phase and vapour phase. Vapour pressure at this stage is known as equilibrium vapour pressure or saturated vapour pressure.
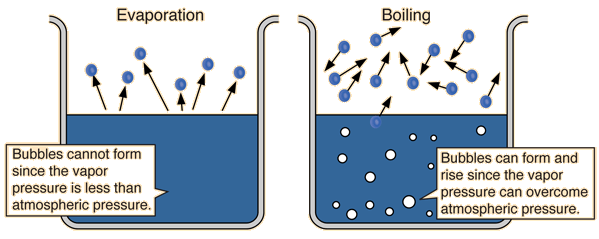
® When a liquid is heated in an open vessel, the liquid vapourises from the surface. At the temperature at which vapour pressure of the liquid becomes equal to the external pressure, vapourisation can occur throughout the bulk of the liquid and vapours expand freely into the surroundings. The condition of free vapourisation throughout the liquid is called boiling. The temperature at which vapour pressure of liquid is equal to the external pressure is called boiling temperature at that pressure.

® At 1 atm pressure boiling temperature is called normal boiling point. If pressure is 1 bar then the boiling point is called standard boiling point of the liquid.
® Boiling does not occur when liquid is heated in a closed vessel. On heating continuously vapour pressure increases. At first a clear boundary is visible between liquid and vapour phase because liquid is more dense than vapour. As the temperature increases more and more molecules go to vapour phase and density of vapours rises. At the same time liquid becomes less dense. It expands because molecules move apart. When density of liquid and vapours becomes the same; the clear boundary between liquid and vapours disappears. This temperature is called critical temperature.
Surface Tension:
® Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the nature of the intermolecular forces. It is denoted by Greek letter g (Gamma). It has dimensions of kg s–2 and in SI units it is expressed as N m–1.
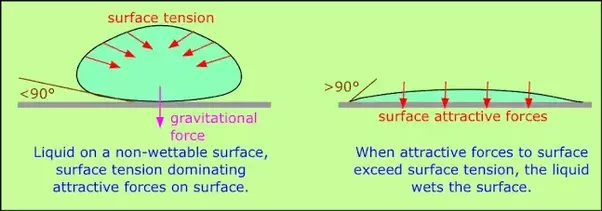
® A typical molecule in the interior of the droplet is surrounded by other molecules that
exert attractive forces from all directions. Consequently, there is no net force on the molecule that would cause it to move in a
particular direction. In contrast, a molecule on the surface experiences a net attraction toward the drop
because there are no molecules on the outside to balance the forces exerted by
adjacent molecules in the interior. Because a sphere has the smallest possible
surface area for a given volume, intermolecular attractive interactions between
water molecules cause the droplet to adopt a spherical shape. This maximizes
the number of attractive interactions and minimizes the number of water
molecules at the surface. Hence raindrops are almost spherical, and drops of
water on a waxed (nonpolar) surface, which does not interact strongly with
water, form round beads.
® Liquids tend to minimize their
surface area. The molecules on the surface experience a net downward force and
have more energy than the molecules in the bulk, which do not experience any
net force. Therefore, liquids tend to have minimum number of molecules at their
surface. If surface of the liquid is increased by pulling a molecule from the
bulk, attractive forces will have to be overcome. This will require expenditure
of energy. The energy required to increase the surface area of the liquid by
one unit is defined as surface energy.
Viscosity:
® Viscosity (η) is the resistance of a liquid to flow. Some liquids, such as gasoline, ethanol, and water, flow very readily and hence have a low viscosity. Others, such as motor oil, molasses, and maple syrup, flow very slowly and have a high viscosity.
® The type of flow in which there is a regular gradation of velocity in passing from one layer to the next is called laminar flow.
®
If
the velocity of the layer at a distance dz is changed by a value du
then velocity gradient is given by the amount ![]() . A force is required to maintain the
flow of layers. This force is proportional to the area of contact of layers and
velocity gradient i.e.
. A force is required to maintain the
flow of layers. This force is proportional to the area of contact of layers and
velocity gradient i.e.
F ∝ A
F ∝ ![]()
F ∝ A × ![]()
F ∝ ηA × ![]()
Where,
F is the viscous force,
A is the area in contact,
![]() is the velocity gradient,
is the velocity gradient,
![]() is a constant called
the coefficient of viscosity.
is a constant called
the coefficient of viscosity.
The SI unit of viscosity coefficient is 1 newton second per square metre
(N s m–2) = pascal second (Pa s = 1 kg m–1s–1).