Bond Dissociation Energy
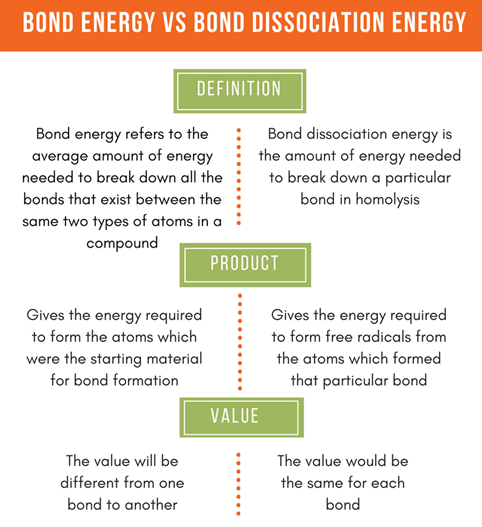
The
bond dissociation energy (enthalpy change) for a bond A B which is broken
through the reaction AB : A B is defined as the standard-state enthalpy
change for the reaction at a specified temperature, here at 298 K.
That is,
ΔHf
298= ΔHf(A)298 +ΔHf
(A)298 -ΔHf
(AB)298
All
values refer to the gaseous state and are given at 298 K. Values of 0 K are
obtained by subtracting 3⁄2RT from the value at 298 K.
To convert the tabulated
values to kcal/mol, divide by 4.184.
|
Bond Dissociation Energies |
|
|
Bond |
Bond
Energy (kj/mol) |
|
H—H |
432 |
|
H—F |
565 |
|
H—Cl |
427 |
|
H—Br |
363 |
|
H—I |
295 |
|
C—H |
413 |
|
C—C |
347 |
|
C—N |
305 |
|
C—O |
358 |
|
C—F |
485 |
|
C—Cl |
339 |
|
C—Br |
276 |
|
C—I |
240 |
|
C—S |
259 |
|
N—H |
391 |
|
N—N |
160 |
|
N—F |
272 |
|
N—Cl |
200 |
|
N—Br |
243 |
|
N—O |
201 |
|
O—H |
467 |
|
O—O |
146 |
|
O—F |
190 |
|
O—Cl |
203 |
|
O—I |
234 |
|
F—F |
154 |
|
F—Cl |
253 |
|
F—Br |
237 |
|
Cl—Cl |
239 |
|
Cl—Br |
218 |
|
Br—Br |
193 |
|
I—I |
149 |
|
I—Cl |
208 |
|
I—Br |
175 |
|
S—H |
347 |
|
S—F |
327 |
|
S—Cl |
253 |
|
S—Br |
218 |
|
S—S |
266 |
|
Si—Si |
340 |
|
Si—H |
393 |
|
Si—C |
360 |
|
Si—O |
452 |
|
C
= C |
614 |
|
C
≡ C |
839 |
|
O
= O |
495 |
|
C
= O |
799 |
|
C
≡ O |
1072 |
|
N
= O |
607 |
|
N
= N |
418 |
|
N
≡ N |
941 |
|
C
≡ N |
891 |
|
C
= N |
615 |