Enthalpy of Reaction
Enthalpies of Reactions:
|
Enthalpies |
Definitions |
Example |
|
Enthalpy of Formation |
Enthalpy change when one mole of a given
compound is formed from its elements |
H2(g) + 1/2O2(g)
→ 2H2O(l),
|
|
Enthalpy of Combustion |
Enthalpy change when one mole of a substance
is burnt in oxygen. |
CH4 + 2O2(g) →
CO2 + 2H2O(l),
|
|
Enthalpy of Neutralization |
Enthalpy change when one equivalent of an
acid is neutralized by a base in dilute solution. |
H+ (aq)
+ OH (aq) → H2O(l)
|
|
Enthalpy of Hydration |
Enthalpy change when a salt combines with
the required number of moles of water to form specific
hydrate. |
CuSO4(s) + 5H2O (l) → CuSO45H2O, ΔhydH°
= 18.69 kcal |
|
Enthalpy of Transition |
Enthalpy change when one mole of a substance
is transformed from one allotropic form to another allotropic form. |
C(graphite) → C(diamond),
ΔtransH°
= 1.9 kJ/mol |
|
Enthalpy of Sublimation |
Enthalpy change when one mole of a solid
substance sublime at constant temp. and 1 bar pressure |
CO2(S) → CO2(g) |
|
Enthalpy of fusion |
Enthalpy change when one mole of a solid
melts |
H2O(S) → H2O
(l) |
'Heat of reaction' or 'Enthalpy of reaction' is a general term used for
the heat change (enthalpy change) accompanying any reaction. However, depending
upon the nature of the reaction (i.e., combustion, neutralization etc.), the
enthalpy of reaction is named accordingly (i.e., enthalpy of combustion,
enthalpy of neutralization etc.). Similarly, depending upon the type of process
involving a phase change such as fusion, vaporization, sublimation etc., the
enthalpy change involved is named accordingly (i.e., enthalpy of fusion,
enthalpy of vaporization etc.).
A few important heats of
reactions are as follows:
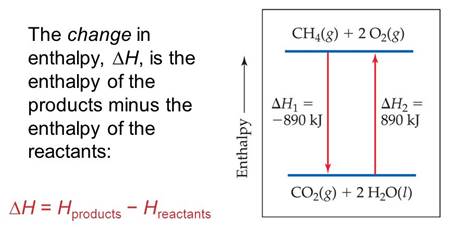
1. Enthalpy of combustion:
The enthalpy of combustion
of a substance is defined as the heat change (usually the heat evolved) when 1
mole of substance is completely burnt or oxidized in oxygen.
CH4 (g) + 202 (g) → CO2
(g) + 2 H20 (g), ∆H = - 890.4 kJ mol-1
This reaction shows that 890.4 kJ of heat is
produced when 1 mole of methane is completely burnt. Hence, enthalpy of
combustion of methane is 890.4 kJ mol-1.
Standard enthalpy of combustion is the amount
of heat evolved when one mole of the substance under standard conditions (298
K, 1 bar pressure) is completely burnt to form products also under standard
conditions. It is represented by ∆CH°
The standard enthalpy of combustion of butane, C4H10
representing the combustion of 1 mole of butane, may be represented as follows:
C4H10 (g)
+ ![]() O2(g) →
4 CO2(g) + 5 H2O (l), ∆CH°= - 2658.0 kJ
(i)
O2(g) →
4 CO2(g) + 5 H2O (l), ∆CH°= - 2658.0 kJ
(i)
Calorific
values of foods and fuels:
Just as the fuels like coal, kerosene oil,
gasoline (petrol), diesel oil etc. are burnt to produce energy for the running
of machines, similarly for the working of the hum carbohydrates, fats etc. in
the form of food. The carbohydrates are first decomposed in our body by the
enzymes to form glucose which then undergoes oxidation by the oxygen that we
inhale to produce energy.
C6H12O6 (s) + 6O2
(g) → 60O2 (g) + 6H20 (g) H° = -
2840.0 kJ mol-1
This oxidation
reaction is usually called 'combustion of food
Different fuels and foods produce different amounts of heat on
combustion. These are usually expressed in terms of their calorific values
which is defined as follows:-
The calorific
value of a fuel or food is the amount of heat in calories or joules produced
from the complete combustion of one gram of the fuel or the food.
2. Enthalpy of formation:
The enthalpy of
formation of a substance is defined as the heat change, i.e., heat absorbed
when 1 mole of the substance is formed from its elements under given conditions
of temperature and pressure. It is usually represented by ∆ Hf.
The conditions of temperature and pressure usually chosen are 298 K and
1 bar pressure. This is called standard state. The enthalpy of formation under
these conditions is called standard enthalpy of formation.
Standard enthalpy of formation of a substance
is defined as the enthalpy change accompanying the formation of 1 mole of the
substance in the standard state from its elements, also taken in the standard
state (i.e., 298K and 1 bar pressure). It is usually represented by ∆ Hf°.
Importance of standard enthalpies of
formation. Knowing the standard enthaplies of the
different compounds involved in a chemical reaction, the standard enthalpy
change of the given can be calculated using the formula*
∆r H° = [Sum of the standard enthalpies of formation of
products of formation] - [Sum of the standard enthalpies of formation of products
of formation of reactants]
i.e., ∆rH°
=∑∆fH° (Products) ∑∆fH° (Reactants)
Thus, for a general reaction, aA + bB → cC + dD
∆rH° = [c ∆fH° (C) + d ∆fH°
(D)] [a ∆fH° (A) + b ∆fH° (B)]
3. Enthalpy of Neutralization
The enthalpy of
neutralization of an acid by a base is defined as the heat change (usually in
heat evolved) when one gram equivalent of the acid is neutralized by a base,
the reaction being carried out in dilute aqueous solution.
The enthalpy of neutralization of a base by an acid
is defined in a similar manner.
For example, when one gram
equivalent of HCI is neutralized by NaOH or one gram
equivalent of NaOH is neutralized by HCl, both solutions being dilute and aqueous, 57.1 kJ of
heat is produced. Thus, we may write :
NaOH + HCI →
NaCl +H2O , ∆neut H = -57.1 kJ mol-1
Hence, enthalpy of neutralization
of HCl with NaOH or NaOH with HCl is 57.1 kJ.
The enthalpy of neutralization of
any strong acid (HCI, HNO3, H2SO4)
with a strong base (LiOH, Naon
KOH) or vice versa, is always the same, i.e., 57.1 kJ. This is because the
strong acids, strong bases and the salts that they form, are all completely
ionized in dilute aqueous solution. Thus, the reaction between any strong ucid and strong. base, e.g., in
the above case may be written as
Na+ +
OH- + H+ + CI- → Na+ + CI-
+ H2O, ∆H = - 57.1 kJ mol-1
H+ (aq) + OH- (aq) → H2O
(2), ∆neut H = - 57.1 kJ
mol-1
Thus, enthalpy of neutralization is the heat
evolved for the reaction between the H+ io
the acid with the OH ions given by the base to form one mole of H2O.
Since strong acids and strong
bases ionize completely in dilute aqueous solution, the number of H+ ions and OH-
ions produced by one gram equivalent of the
strong acid and the strong base is always the same. Hence, the enthalpy of
neutralization between a strong acid and a strong base is always
constant.
In case either the acid or the base or both
are weak, the enthalpy of neutralization is
usually less than 57.1 kJ. The reason for this may be understood by considering
the neutralization of a weak acid with a strong base like NaOH . Acetic
acid ionizes to a small extent whereas NaOH ionizes
completely as
The reason with a strong by
acid with base.
(i)
CH3COOH ⇌ CH3COO- + H+
(ii)
NaOH →
Na+ + OH-
When H+ ions given by
the acid combine with the OH ions given by the base, the equilibrium (i) shifts to the right, i.e., more of acetic acid
dissociates. A part of the heat produced during the combination of H+
ions and OH- ions is used up for the complete dissociation of acetic
acid. The heat thus used up is called enthalpy of dissociation or enthalpy of
ionization. It is 1.9 kJ for the acetic acid. Hence, the net heat evolved in
the above reaction is 57.1 1.9 = 55.2 kJ.
Similarly,
in the neutralization of NH4OH with HCl,
5.6 kJ of heat is used up for the dissociation of the weak base, i.e., NH, OH.
Hence, the enthalpy of neutralization in this case is only 57.1 5.6 = 51.5
kJ.
4. Enthalpy of Solution:
The enthalpy of solution
of a substance in a particular solvent is defined as the enthalpy change (i.e.,
amount of heat evolved or absorbed) when 1 mole of the substance is dissolved
in a specified amount of the solvent. However, if such a large volume of the
solvent is taken that further addition of the solvent does not produce any more
heat change, it is called enthalpy of solution at infinite dilution.
Water is
usually used as the solvent and the symbol aq
(aqueous) is used to represent it at large dilutions (infinite dilutions).
Thus, the thermochemical equations for the dissolution of KCl
and CuSO4 may be represented as :
KCl (s) + aq
→ KCl (aq), ∆sol
H = +18.6 kJ mol-1
CuSO4
(s) + aq → CuSO4 (aq), ∆sol H = - 66.5 kJ mol-1
Thus, the first case is
endothermic and enthalpy of solution = + 18.6 kJ mol-1
The second case is exothermic and
enthalpy of solution = - 66.5 kJ mol-1
It is interesting to note that
the salts like copper sulphate, calcium chloride
etc., when present in the hydrated state (i.e., CuSO4.5H2O,
CaCl2.6H20 etc.) dissolve with the absorption of heat.
For example,
CuSO4.5H2O
+ aq → CuSO4
(aq), ∆sol H = + 11.7 kJ
Thus, it can be generalized that
the process of dissolution is usually endothermic for
(i)
Salts which do not form hydrates like NaCl, KCI, KNO, etc.
(ii)
Hydrated salts like CuSO4.5H20,
CaCl2.6H20 etc.
5. Enthalpy of atomization
:
When one
mole of a given substance dissociates into gaseous atoms, the enthalpy change
accompanying the process is called enthalpy of atomisation.
It is represented by the symbol ∆aH°.
For example,
H2 (g) →
2 H (g), ∆aH° = 435.0 kJ mol-1
CH4 (g) →C
(g) + 4 H (g), ∆aH° = 1665 kJ mol-1
Na (s) → Na (g), ∆aH°
= 108.4 kJ mol-1
In the first example, enthalpy of atomization
is same as bond dissociation enthalpy (discussed later). In the third example,
enthalpy of atomisation is same as enthalpy of
sublimation. The second reaction represents only enthalpy of atomisation and not bond energy as discussed later.
6. Enthalpy of ionization:
When one mole of a
covalent compound on dissolution in water splits to produce ions in the
solution, the enthalpy change accompanying the process is called enthalpy of ionisation.
For example,
HCI (g) + aq →
H+ (aq) + CI- (aq),
∆ion H° = 75.2 kJ mol-1
Thus,
enthalpy of ionization of HCl (g) is 75.2 kJ mol-1.
In fact, the enthalpy of ionization of a covalent compound is the same as its
enthalpy of solution or enthalpy of dissolution (∆solution
H°).
7. Enthalpy of formation of ions
When an ionic solid is dissolved in Water,
free ions are produced in the aqueous solution.
For the calculation of formation of an ion in the aqueous solution, enthalpy of
formation of H+ jon in the aqueous solution is taken as zero.
For example,
∆fH° for chloride ion in aqueous solution can be calculated from the following data :
![]() H2 (g)+
H2 (g)+ ![]() C12 (g) → HCI (g), ∆fH° = - 92.8 kJ mol-1
C12 (g) → HCI (g), ∆fH° = - 92.8 kJ mol-1
HCI (g) + aq → H+
(aq) + CI- (aq),
∆dissH° = - 75.2 ∆fH° [CI-
From eqn.
(ii), ∆rH°
= {∆fH° [H+ (aq)] + ∆fH° [CI- (aq)]}
- ∆fH° (HCI)
∴ - 75.2 = 0 -∆fH° [CI- (aq)] - (- 92.8)
Or
∆fH° [CI- (aq)]=-75.2-92.8=-168.0 kJ mol-1
Similarly,
enthalpy of formation of OH- ions can be calculated from enthalpy of
formation of H2O as H+ + OH→H20,
∆H = - 13.7 kcal.
8. Enthalpy of Hydration:
The amount of
enthalpy change (i.e., the heat evolved or absorbed) when one mole of the
anhydrous salt combines with the required number of moles of water so as to
change into the hydrated salt, is called the enthalpy of hydration or heat of
hydration.
For example, the enthalpy
of hydration of copper sulphate is -78.2 kJ mol-1 . This may be represented
as
CuSO4 (s) + 5 H2O → CuSO4.5H20
(s), ∆hydH = -78.2 kJ mol-1.
9. Enthalpy of Hydrogenation
The amount of
enthalpy change that takes place when one mole of an unsaturated organic
compound is completely hydrogenated is called enthalpy of hydrogenation.
For
example, enthalpy of hydrogenation of ethylene is the enthalpy change for the
reaction,
CH2
= CH2 + H2 → CH3 CH3, ∆H = ∆Hhyrogenation
10. Enthalpy of Allotropic
Transformation
The enthalpy change
that takes place when one mole of one form of an allotropic modification
changes to another is called enthalpy of allotropic transformation.
For example,
C (diamond) → C
(diamond), ∆H = ∆Htransform
S (Monoclinic) → S (Rhombic), ∆H = ∆Htransform
11. Heat of Dilution
The increase in enthalpy accompanying the addition
of a specified amount of solvent to a solution of constant pressure. Also known
as integral heat of dilution; total heat of dilution.
The increase in enthalpy when an infinitesimal
amount of solvent is added to a solution at constant pressure. Also known as
differential heat of dilution.
Enthalpy Changes During Phase
Transitions
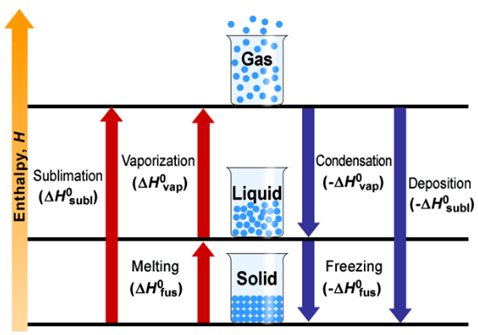
1. Enthalpy of Fusion
Enthalpy of fusion
is the enthalpy change accompanying the transformation of one mole of a solid
substance into its liquid state at its melting point. It is also called molar
enthalpy of fusion.
For example, the molar enthalpy of fusion (∆fusH)
of ice (m.p. =273 K) is 6.0 kJ mol-1
It may be represented as :
H20 (s) →H20 (l), ∆fusH = + 6.0 kJ mol-1
Ice Liquid Water
Water as freezing is reverse of fusion, the enthalpy
of freezing (or enthalpy of solidification value as the enthalpy of fusion but
has the opposite sign. Thus,
H20 (l) → H20 (s), ∆freezingH = - 6.0 kJ mol-1
Liquid water Ice
2. Enthalpy of Vaporisation
It is the amount of
heat required to convert one mole of a liquid into its vapour
state at its boiling point. It is also called molar enthalpy of vaporisation.
For
example, the molar enthalpy of vaporisation (∆vapH) of water into its vapour (steam) at the boiling point of water (373 K) is
40.7 kJ. It may be represented as
H20 (l) → H2O (g), ∆vapH
= + 40.7 kJ mol-1
Water Steam
As condensation
is reverse of vaporisation, the enthalpy of
condensation has the same value as the enthalpy of vaporisation
but has opposite sign. Thus,
H2O (g) → H20 (l), ∆condH
= 40.7 kJ mol-1 .
Steam
Water
3. Enthalpy of Sublimation
Sublimation is a process in which a solid on heating changes directly
into gaseous state below its melting point.
Enthalpy of
sublimation of a substance is the enthalpy change accompanying the conversion
of 1 mole of a solid directly into vapour phase at a
given temperature below its melting point.
∆subH = ∆fusH + ∆vapH
Otherwise also; this equation is true because
enthalpy is a state property.
The
magnitude of enthalpy change for a phase transition depends upon the strength
of intermolecular forces, e.g.,
∆vapH for H2O is much larger
than that for acetone because the former has intermolecular hydrogen bonding.
Problems:
1. If C+O2→CO2 + 94.2kcal
H2+12O2→H2O + 68.3kcal
CH4+2O2→CO2+2H2O + 210.8kcal
then the possible heat of methane will be
A. 47.3
kcal
B. 20.0
kcal
C.
45.9 kcal
D. 47.3
kcal
Solution:
C+O2
→ CO2+94.2Kcal. (i)
H2+12O2
→ H2O+68.3Kcal. (ii)
On multiplication of eq. (ii) by 2 and
then adding in eq. (i)
C+2H2+2O2
→ CO2+2H2O+230.8Kcal (iii)
On
subtracting eq. (iii) by following eq.
CH4+2O2
→ CO2+2H2O+210.8Kcal.
We get,
C+2H2
→ CH4
ΔH=20Kcal.
2. The enthalpy of
fusion of ice per mole
A. 18
kJ
B. 8
kJ
C.
80 kJ
D. 6
kJ
Solution:
The
enthalpy of fusion of ice per mole 6 kJ
3. In which of the
following neutralisation reactions, the heat of neutralisation will be highest
A. NH4OH and CH3COOH
B. NH4OH
and HCl
C.
NaOH and CH3COOH
D.
NaOH and HCl
Solution:
Heat of neutralisation between strong acid and a strong base is
about −13.7Kcal.
4. From Kirchhoff's
equation which factor affects the heat of
reaction
A. Pressure
B. Temperature
C.
Volume
D. Molecularity
Solution:
Effect of temperature
in heat of reaction is given by Kirchoffs equation.
5. The molar neutralization heat for KOH and HNO3 as compared to molar
neutralization heat of NaOH and HCl
A. Less
B. More
C.
Equal
D. Depends
on pressure
Solution:
Heat of neutralisation
between strong acid and a strong base is about −13.7Kcal.
6. An exothermic
reaction is one in which the reacting substances
A. Have
more energy than the products
B. Have
less energy than the products
C.
Are at a higher temperature than
the product
D. None
of the above
Solution:
For exothermic reactions HP<HR.
For endothermic reactions HP>HR.
7. Which of the
following statement is correct?
A. ΔH is
positive for exothermic reaction
B. ΔH is
negative for endothermic reaction
C.
The heat of neutralization of
strong acid and strong base is always the same
D. The
enthalpy of fusion is negative
Solution:
ΔH=−ve for
exothermic reaction.
ΔH=+ve for
endothermic reaction
Enthalpy of fusion is + ve.
8. If the enthalpy of B is greater than of A, the
reaction A→B is
A. Endothermic
B. Exothermic
C.
Instantaneous
D. Spontaneous
Solution:
For exothermic reactions Hp<HR.
For endothermic reactions Hp>HR.
9. Enthalpy change for
reaction, 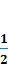 H2+
H2+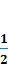 Cl2→HCl, is called
Cl2→HCl, is called
A. Enthalpy
of
combination
B. Enthalpy
of reaction
C.
Enthalpy of
formation
D. Enthalpy
of fusion
Solution:
Enthalpy of formation of HCl.
10. The enthalpy of
neutralization is about 57.3 kJ for the pair
A. HCl and NH4OH
B. NH4OH and HNO3
C.
HCl and NaOH
D.
CH3COOH and NaOH
Solution:
Heat of neutralisation
between strong acid and a strong base is about −13.7Kcal.
11. The heat of reaction does not depend upon
A. Temperature
of the reaction
B. Physical
state of reactants and products
C. Whether
the reaction is carried out at constant pressure or at constant volume
D. The
method by which the final products are obtained from the reactants
Solution:
The heat of reaction does not depend
upon the method by which the final
products are obtained from the reactants
12. Heat of neutralisation of a strong acid by a
strong base is a constant value because
A. Salt
formed does not hydrolyse
B. Only H+ and OH− ions react in every case
C.
The strong base and strong acid
react completely
D. The
strong base and strong acid react in aqueous solution
Solution:
In neutralisation
of a strong acid and base only H+and OH− ions
react.
13. Heat of neutralisation of an acid by a base is highest when [KCET
1985]
A. Both
the acid and base are weak
B. Both
the acid and base are strong
C.
The acid is strong and the base is
weak
D. The
acid is weak and the base is strong
Solution:
When
both acid and base are strong then heat of neutralisation
is 57.1kJmol−1
14. Heat of combustion of a substance
A. Is
always positive
B. Is
always negative
C. Is
equal to heat of formation
D. Nothing
can be said without reaction
Solution:
Heat of combustion of a substance is
always negative
15. The heats of combustion of rhombic and monoclinic sulphur
are respectively 70960 and 71030 calories. What will be the heat of conversion
of rhombic sulphur to monoclinic
A. 70960
calories
B. 71030
calories
C.
70
calories
D. +
70 calories
Solution:
S (rhombic) +O2→SO2, ΔH=70960cal. ...(i)
S (monoclinic) +O2→SO2ΔH=71030cal
(ii)
Aim: S (rhombic) → S
(monoclinic)
eq. (i) - eq. (ii) gives the required result.
16. Which of the following fuels will have
the highest calorific value (kJ/kg)
A. Charcoal
B. Kerosene
C. Wood
D. Dung
Solution:
Out of given substances, kerosene oil has maximum calorific value.