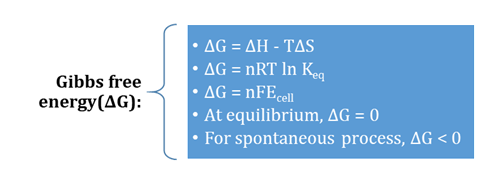
Gibbs Free Energy
It is another thermodynamic quantity that
helps in predicting the spontaneity of a
process, earlier it was called as Gibbs free energy but according to
IUPAC
system, now it is simply called as Gibbs energy or Gibbs function. However the
use of the term 'free energy' has not yet been completely stopped.
Gibbs Free Energy Changes in Reactions
It is usually denoted by ‘G’ and is defined mathematically by the
equation,
G=H-TS
where H is the heat content, T
is the absolute temperature and S is the entropy of the system.
As before, for the isothermal
processes, we have
G1 = H1 – TS1 for the initial state
G2 = H2 – TS2
for the final state
G2 - G1 = (H2 - H1)
– T (S2-S1) or ∆G=∆H - T∆S
where
∆G = G2
– G1 is the change in Gibbs's free energy of the system
∆H = H2
– H1 is the enthalpy change of the system and
∆S = S2
- S1 is the entropy change of the system.
The equation, ∆G=∆H-T∆S, is known as
Gibbs-Helmoholtz equation (or simply Gibbs energy
equation).
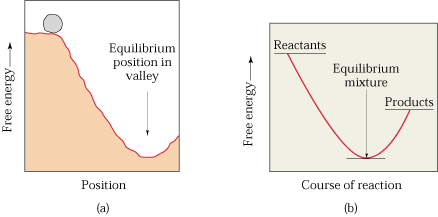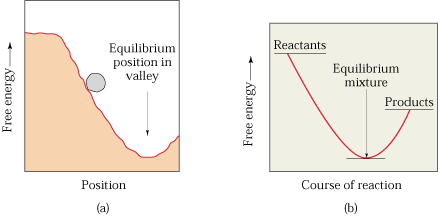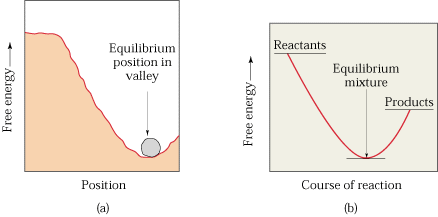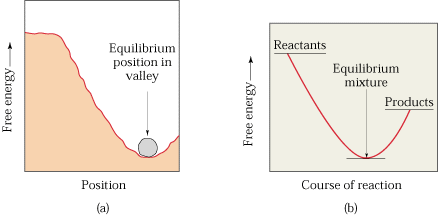
Spontaneity In Terms of Free Energy Change
(a) Deriving the
criteria from entropy considerations
It has already been explained that the total entropy change when the
system is not isolated from the surroundings is given by
∆Stotal = ∆Ssystem + ∆Ssurroundings ...(i)
Consider a process (or a reaction) being carried out at constant
temperature and pressure. Suppose the heat is lost by the surroundings and
gained by the system. If the heat lost by the surroundings is represented by qp (p indicating that
the process is being carried out at constant pressure), then by definition of
entropy change
∆Ssurroundings =![]() ...(ii)
...(ii)
(minus sign before qp indicates
that the heat is lost by the surroundings). Further, we know that at constant
pressure,
qp =∆H ...(iii)
Substituting
this value in eqn. (ii), we get
∆S surroundings = ![]() ...(iv)
...(iv)
Substituting
this value in eqn. (i), we get
∆S total
= ∆S system - ![]() ...(iv)
...(iv)
Using the symbol
∆S in place of ∆S system (being implied that ∆S, stands for ∆S for the system), we can write eqn.
(iv) as
∆S total = ∆S - ![]() ...(v)
...(v)
Multiplying throughout by T, we get
T∆Stotal= T∆S – ∆H …(vi)
But for a change taking place at constant temperature and pressure,
∆G=∆H - T∆S ...(vii)
Substituting
this value in eqn. (vi), we get
T∆Stotal = - ∆G or ∆G
= - T∆Stotal ...(viii)
But in terms of total entropy change, it has already
been explained that
if
∆Stotal is positive, the process is
spontaneous
if
∆S total is zero, the process is in equilibrium, and
if
∆Stotal, is negative, the direct
process is non-spontaneous the reverse process may be spontaneous.
Putting
these results in eqn. (viii), it can be concluded that the criteria in terms of
use for the spontaneity of the process will be as follows :
(i)
If AG is negative, the
process will be spontaneous.
(ii)
If AG is zero, the
process is in equilibrium.
(iii)
If AG is positive, the
direct process is non-spontaneous; the reverse process may be spontaneous.
An important advantage of free energy criteria
over the entropy criteria lies in the fact that the rommet
requires free energy change of the system only whereas the latter requires the
total entropy change for the system and the surroundings.
(b) Deriving the criteria from Gibbs energy equation
According to Gibbs energy equation,
∆G=∆H - T∆S
This equation
combines in itself both the factors which decide the spontaneity of a process,
namely,
(i)
the
energy factor, ∆H
(ii)
the
entropy factor, T∆S
Thus, ∆G
is the resultant of the energy factor (i.e., tendency for minimum energy) and
the entropy factor (i.e., the tendency for maximum randomness).
Depending upon the signs of ∆H and T∆S and their relative magnitudes, the following different possibilities
arise :
I.
When
both ∆H and T∆S are negative, i.e., energy factor favours the process but randomness factor opposes it. Then
(i)
If ∆H
> T∆S the process is spontaneous and AG is negative.
(ii)
If ∆H
< T∆S, the process is
non-spontaneous and AG is positive.
(iii)
If ∆H=
T∆S, the process is in
equilibrium and AG is zero.
II.
When
both ∆H and T∆S are positive, i.e., energy factor opposes the
process but randomness factor favours it. Then
(i)
If ∆H
> T∆S , the process is non-spontaneous and AG is
positive.
(ii)
If ∆H
< T∆S , the process is spontaneous and AG is
negative.
(iii)
If ∆H
= T∆S , the process is in equilibrium and AG is
zero. III. When AH is negative but TAS is positive, i.e., energy factor as well
as the randomness factor
III.
When ∆His
negative but T∆S is positive i.e., energy factor as well as randomness
factor favour the process. The process will be highly
spontaneous and ∆ G will
be highly negative.
IV.
When ∆H is positive but T∆S is negative, i.e.,
energy factors as well as the randomness factor oppose the process. The process
will be highly non-spontaneous and AG will be highly positive.
To sum up, the criteria for spontaneity of a
process in terms of AG is as follows:
(i)
If AG is negative the process
is spontaneous.
(ii)
If AG is zero, the
process does not occur or the system is in equilibrium.
(iii)
If AG is positive the process does not occur
in the forward direction. It may occur in the forward direction. It
may occur in the backward direction.
Problems
1. Which of the following
pairs of a chemical reaction is certain to result in spontaneous reaction?
A. Exothermic and decreasing disorder
B. Endothermic and increasing
disorder
C. Exothermic and increasing disorder
D. Endothermic and decreasing
disorder
Solution:
ΔG=ΔH−TΔS
For spontaneous
process ΔG should be negative in option (3)
ΔH=−ve and ΔS=+ve then
ΔG=
(−ve) −T (+ve)
=−ve
2. Which
of the following endothermic processes are spontaneous
A. Melting
of ice
B. Evaporation
of water
C.
Heat of
combustion
D. Both
A and B
Solution:
Both Melting of
ice and Evaporation of water
are
spontaneous
3. The free energy change for a
reversible reaction at equilibrium is
A. Large positive
B. Small negative
C. Small positive
D. 0
Solution:
ΔG at equilibrium = 0.
4. For a spontaneous change, free
energy change ΔG is
A. Positive
B. Negative
C. Zero
D. Can be positive or negative
Solution:
For spontaneous change ΔG=−ve
5. A minus
sign of the free energy change denotes that
A. The reaction tends to proceed
spontaneously
B. The reaction is non-spontaneous
C. The system is in equilibrium
D. The reaction is very much unlikely
Solution:
When ΔG=−ve than
the reaction is spontaneous in nature.
6. The relation between ΔG and ΔH is
A. ΔH=ΔG−TΔS
B. ΔG=ΔH−TΔS
C. TΔS−ΔG=ΔH
D.
ΔH=TΔG+ΔS
Solution:
The relation between ΔG and ΔH is ΔG=ΔH−TΔS
7. The
relation ΔG=ΔH−TΔS was given by [MP PMT 2000; KCET 2002]
A. Boltzmann
B. Faraday
C. Gibbs’Helmholtz
D. Thomson
Solution:
The relation ΔG=ΔH−TΔS was given by Gibbs’
Helmholtz
8.
For precipitation reaction of Ag+ ions with NaCl, which of the following statements is
correct?
A.
ΔH for the reaction is zero
B.
ΔG for the reaction is zero
C.
ΔG for the reaction is negative
D.
[ΔG]=[ΔH]
Solution:
Because the reaction is spontaneous.
9. At constant pressure and temperature, the direction
to the result of any chemical reaction is where, there is less amount of
A. Entropy
B. Enthalpy
C. Gibb's free energy
D. None of the above
Solution:
ΔG = -ve.
10. What is the free energy change ΔG when 1.0mole of water at 100°C and 1atm pressure is converted into steam t 100°C and 1atm pressure
A.
540cal
B.
−9800cal
C.
9800cal
D.
0cal
Solution:
At equilibrium ΔG=0.
11. ΔG° for the reaction X+Y⇌ Z
is - 4.606 kcal. The value of equilibrium constant of the reaction at 227°Cis (R=2.0cal.mol−1K−1)
A. 100
B. 10
C. 2
D. 0.01
Solution:
ΔG°=−2.303RTlogK
−4.606=−2.303×0.002×500 logK
log k =2,
K=100.
12. Spontaneity of a chemical
reaction is decided by the negative change
A. Internal energy
B. Enthalpy
C. Entropy
D. Free energy
Solution:
Spontaneous change shows ΔG=−ve
13. The
essential condition for the feasibility of a reaction is that
A. The reaction should be exothermic
B. The entropy of products must be
larger than that of reactants
C. The reaction is to be accompanied
with free energy decrease
D. The reaction has to possess high
activation energy
Solution:
If ΔG=−ve reaction
is spontaneous.