Internal Energy
Whenever some process occurs, it is usually accompanied
by some energy change. The energy may appear in different forms such
as heat, light, work etc.
The evolution or absorption of energy in different processes clearly
shows that every substance (or the system containing one or more substances)
must be associated with some definite amount of
energy, the actual value of which depends upon the nature of the substance and
the conditions of temperature,
pressure, volume and composition.
It is the sum of different types of energies associated with atoms and
molecules such as electronic energy (Ee),
nuclear energy (En), chemical bond energy (Ec),
potential energy (Ep) and kinetic energy (Ek)
which is further the sum of translational energy (Et), vibrational
energy (Ev) and
rotational energy (Er). It is usually
represented by the symbol 'U' or 'E'.
Thus,
U
or E = Ee + En +Ec +Ep
+ Ek + Et + Ev + Er
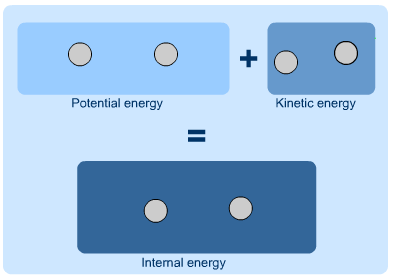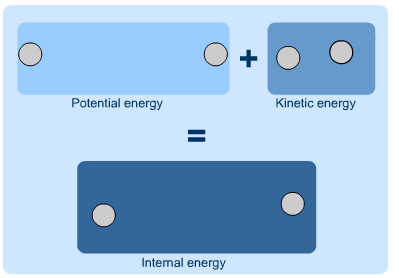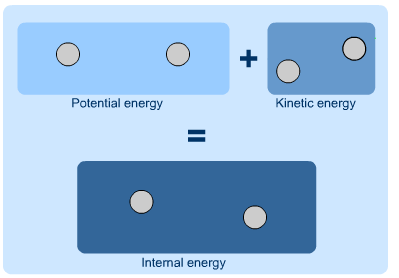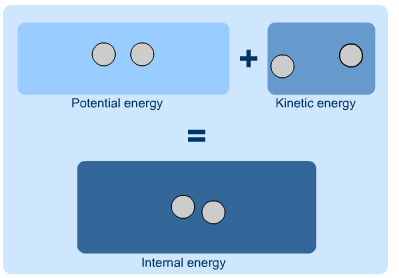
The energy thus stored within a substance (or a
system) is called internal energy.
Sign of ∆U
Obviously, if U1 > U2 (or UR> UP),
the extra energy possessed by the system in the initial state (or the
reactants) would be given out and ∆U will be negative according to the above
equations energy will be absorbed in the process and ∆U
will be positive. Similarly, if U1 <U2 (or UP < UR),
energy will be absorbed in the process and ∆U will be positive. Hence, ∆U is negative if energy is
evolved and ∆U is positive if energy is absorbed.
Units
of U or E.
The units of energy are ergs (in CGS units) or joules (in SI units)
1 joule = 107
ergs.
Problems
1. ∆E is always positive when
(a) System absorbs heat and work is done on it
(b) System emits heat and work is done on it
(c) System emits heat and no work is done on it
(d) System absorbs heat and work is done by it
Solution:
Heat absorbed increases the internal energy and also work done on a
system increases the internal energy.