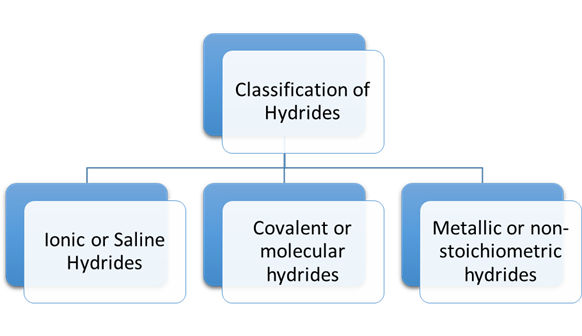
Classification of Hydrides
Hydride Definition:
Dihydrogen, under certain reaction conditions,
combines with almost all elements except noble gases to form binary compounds
called hydrides.
If ‘E’ is the symbol of an element then hydride can be
expressed as e.g., MgH2 or B2H6.
The hydrides are classified into three categories:
1.
Ionic
2.
Covalent
or molecular hydrides
3.
Metallic
or non-stoichiometric hydrides
1. Ionic or Saline Hydrides:
Potassium hydride
What is an Ionic Hydride?
These are stoichiometric compounds of dihydrogen
formed with most of the s-block
elements which are highly electropositive in character.
How they are formed?
·
They are formed by the transfer of electrons from the
metals to the hydrogen atoms.
·
It can be only formed from highly electropositive
element of group 1 and group 2 (except Be and Mg).
General Expression of Preparation:
For the hydrides of group A,
|
2M (g) + H2 (s) |
For the hydrides of group B,
|
M (g) + H2 (g) |
Properties of Ionic Hydrides:
·
In fact
BeH2 and MgH2 are polymeric
in structure.
·
The
ionic hydrides are crystalline,
non-volatile and nonconducting in solid state. However, their melts conduct
electricity.
·
They are thermally very stable.
·
They have high melting and boiling point.
·
They are soluble in water.
·
They are insoluble in organic compounds.
·
They are conductors (electrolytes) of electricity.
·
On
electrolysis liberate dihydrogen gas at anode which confirms the existence of
H– ionc or saline or salt like hydrides.
Uses of Ionic Hydrides:
·
When ionic hydrides react with water
the metal combines with the OH from the water to form a hydroxide salt.
·
The hydrogen from the ionic hydride
and the other water hydrogen combine to form the hydrogen gas.
·
Saline
hydrides react violently with water producing dihydrogen gas.
·
On heating ionic hydrides decompose
to evolve dihydrogen which ignites spontaneously therefore they are used
as solid fuels.
2. Covalent or Molecular Hydride:

Boron Hydride
What is a Covalent Hydride?
Covalent hydrides are primarily compounds of hydrogen
and non-metals in which the bonds are evidently electron pairs shared by atoms
of comparable electronegativities. For example, most nonmetal hydrides are
volatile compounds held together in the condensed state by relatively
weak vanderwaal’s intermolecular interactions.
How they are formed?
·
Covalent
hydrides can be formed from boron (B), aluminum (Al) and gallium (Ga) of group 13 in the periodic
table.
·
Boron
forms an extensive series of hydrides.
Preparation:
·
By direct combination
of elements with dihydrogen:
|
H2 (g)
+ S (l) |
·
By reduction of a
suitable halide with LiAlH4 in dry ether:
|
SiCl4 + LiAlH4 |
·
By hydrolysis of
metal borides, carbides, nitrides, phosphide:
|
Ca3P2 (s) +6 H2O (l)
|
·
By action on suitable
binary compounds:
|
FeS + H2SO4 |
·
By action of an oxo
acids with NaBH4 in aqueous solution:
|
4H3AsO3 + 3NaBH4 |
Properties of
Covalent Hydrides:
·
Covalent hydrides are
usually volatile compounds having low melting and boiling point and also do not conduct electricity.
·
Hydrides of group 13
(BH3, AlH3) do not have sufficient number of electrons to
form normal covalent bond and hence are called electron deficient hydrides. They exist in polymeric forms
such as B2H6, B4H10, etc.
·
Hydrides of group 14
(CH4, SiH4, SnH4, PbH4) have exact
number of electrons to form normal covalent bond and hence are called electron exact or electron precise
hydrides. Their Bond length increases from CH4 to PbH4 as
the size of the element increases from C to Pb.
·
Hydrides of group 15,
16 and 17 (NH3, PH3, H2O, H2S, HF,
HCl) have more electrons than required to form normal covalent bond and hence
are called electron rich hydrides.
·
Group 15 hydrides
have one lone pair, group 16 hydrides have two lone pair, group 17 hydride have
3 lone pair of electrons.
·
The hydrides of first
elements of group 15, 16 and 17 have abnormally high boiling point as compared
to boiling point of the hydrides of second element of each group.
·
The boiling point of
the hydrides of the rest of the elements of each group increases as the atomic
number or the molecular mass of the hydride increases down the group.
·
The lighter elements
of group 14 ,15 and 16 form polynuclear hydrides in which two or more atoms of
same elements are linked together. This property of self-linking of atoms is
called catenation.
Uses of
Covalent Hydrides:
·
A particularly important
segment of covalent hydrides are complex metal hydrides, powerful soluble hydrides commonly used in
synthetic procedures.
·
Hydrides that are soluble
in common solvents are widely used in organic synthesis, particularly common
are sodium borohydride (NaBH4)
and lithium aluminium hydride and hindered reagents such as DIBAL.
3. Metallic or
Non-Stoichiometric Hydrides:
What is Covalent
Hydride?
These are the hydrides of
transition elements (except elements of group I-B and II-B). In transition
elements there are small empty spaces among the atoms. Hydrogen gas adsorbs in
these empty spaces to produce metallic hydrides. These hydrides are also known
as Interstitial hydrides.
How they are formed?
Hydrides of this type form according to either one of two main
mechanisms:
·
The first mechanism involves the adsorption
of dihydrogen succeeded by the cleaving of the H-H bond, the delocalisation of
the hydrogen's electrons and finally, the diffusion of the protons into the
metal lattice.
·
The other main mechanism involves the
electrolytic reduction of ionised hydrogen on the surface of the metal lattice,
also followed by the diffusion of the protons into the lattice.
Preparation:
These hydrides are generally formed by,
·
Transition metals of group 3, 4, 5 of d- block,
·
Cr metal of group 6 and
·
f-block elements.
Properties of
Metallic Hydrides:
·
They are generally
powders or brittle solids having dark or metallic appearances.
·
They are good
conductors of electricity. The conductivity decreases with increase in
temperature.
·
They have high
thermal conductivity.
·
Most of these hydrides are harder than parent
metals.
·
They generally undergo reversible
decomposition into H2 gas and metal.
·
They do not follow
stoichiometric rules.
·
They behave like pure
metal.
·
Bonding between metal and
hydrogen is metallic bond.
·
On heating they release
hydrogen in atomic state.
·
They are solids.
·
They are not true chemical compounds. They are used
as reducing agent in different processes.
·
There properties are between ionic hydrides and
covalent hydrides.
Uses of
Metallic Hydrides:
·
Metal
hydrides are often used in fuel cell applications that use hydrogen as a fuel.
·
Nickel
hydrides are often found in various types of batteries particularly NiMH
batteries.
·
Nickel
metal hydride batteries rely on hydrides of rare earth intermetallic compounds,
such as lanthanum or neodymium bonded with cobalt or manganese.
·
Lithium
hydrides and sodium borohydride both serve as reducing agents in chemistry
applications.